A profound biological mystery, the condition of microcephaly—characterized by an abnormally small developing brain—has long puzzled medical science, particularly when it affects children. Addressing this complex question, a collaborative international research initiative, involving esteemed institutions such as the German Primate Center, Hannover Medical School, and the Max Planck Institute of Molecular Cell Biology and Genetics, has employed cutting-edge human brain organoid technology. These sophisticated laboratory-grown models have provided an unprecedented window into the intricate mechanisms by which disruptions in fundamental cellular structural proteins can arrest or impede the early stages of cerebral development.
The groundbreaking research, detailed in the scientific journal EMBO Reports, elucidates how genetic alterations affecting actin, a critical component of the cellular scaffolding, profoundly interfere with the fundamental process of cell division in nascent brain progenitor cells. When these crucial cells are unable to replicate and divide with the requisite precision and frequency, their overall numbers are diminished, directly limiting the potential for expansive brain growth and ultimately leading to a smaller cranial volume. As stated by Indra Niehaus, the lead author of the investigation and a research associate at Hannover Medical School, "Our discoveries offer the inaugural cellular-level explanation for microcephaly observed in individuals diagnosed with the infrequent Baraitser-Winter syndrome."
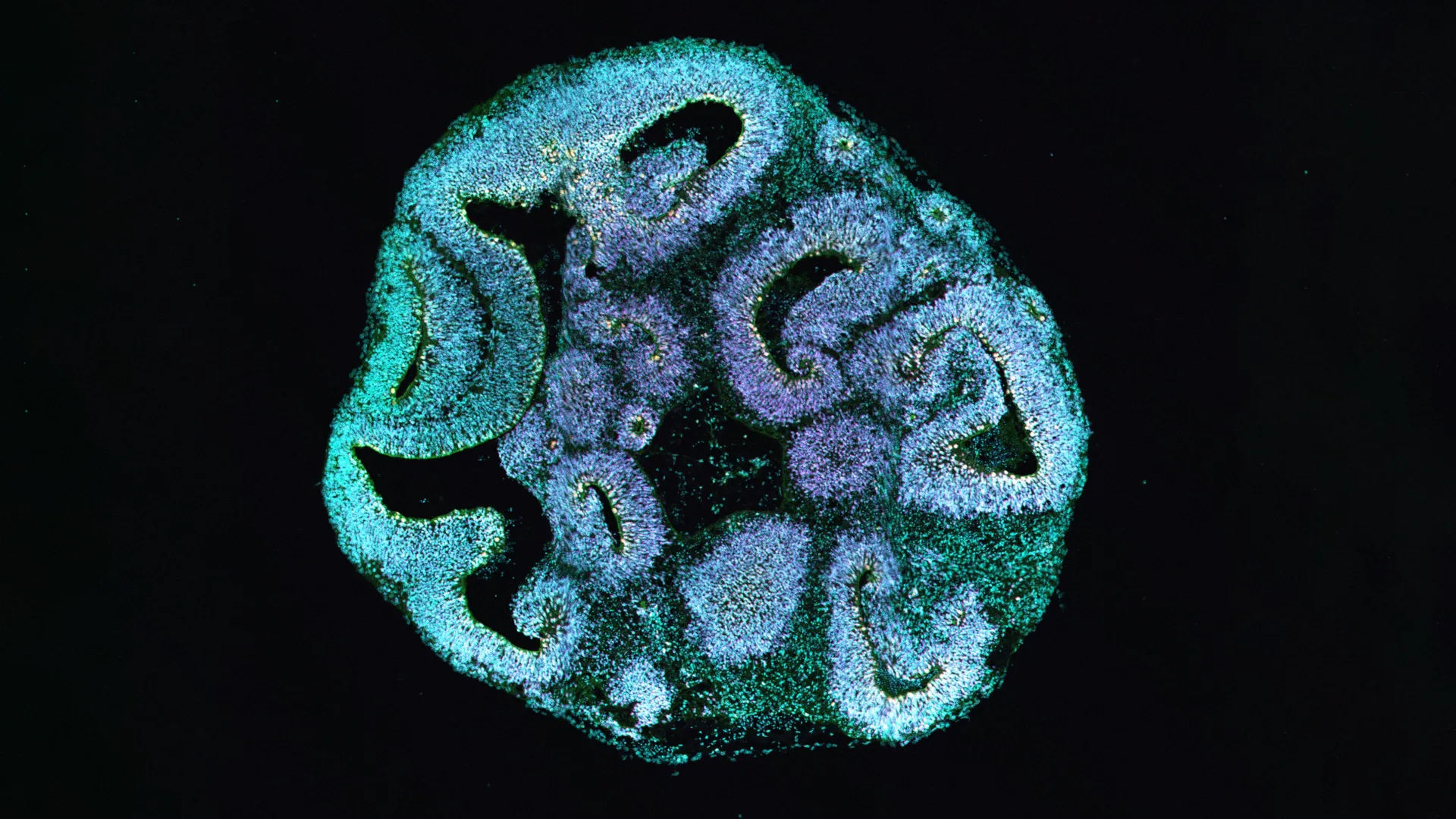
The intricate internal framework of a cell, known as the cytoskeleton, plays an indispensable role in dictating cell shape, providing structural integrity, and facilitating the internal transport of vital molecules. Actin is a cornerstone protein within this dynamic network. In individuals afflicted with Baraitser-Winter syndrome, a specific mutation within one of two pivotal actin genes is implicated. To meticulously investigate the downstream consequences of this genetic anomaly, the research team embarked on a process of cellular reprogramming. They transformed skin cells obtained from patients exhibiting the syndrome into induced pluripotent stem cells, which possess the remarkable ability to differentiate into various cell types. These reprogrammed stem cells then served as the foundation for cultivating three-dimensional brain organoids, designed to meticulously replicate the nascent phases of human brain formation.
Following an observational period of thirty days of developmental progression within these organoids, a striking divergence became apparent. The brain organoids derived from the cells of affected patients exhibited a measurable size deficit, being approximately 25 percent smaller when compared to organoids cultivated from the cells of healthy donors. Further microscopic examination revealed that the ventricle-like spaces within these organoids, which are the specialized regions where progenitor cells proliferate and initiate the formation of primitive neurons, were also significantly reduced in size.
A detailed analysis of the cellular composition within these organoids unveiled a distinct and critical imbalance in key brain cell populations. The number of apical progenitor cells, which are absolutely indispensable for the construction of the cerebral cortex—the brain’s outermost layer responsible for higher-level cognitive functions—was found to be substantially diminished. Concurrently, there was a notable increase in the population of basal progenitor cells. These cell types typically emerge at later stages of developmental progression, suggesting a premature shift in cellular fate and differentiation.
This observed alteration in cell type proportions strongly indicated that the fundamental timing and the ultimate outcome of progenitor cell division had been significantly disrupted. This disruption, the researchers posited, is a primary contributor to the observed failure of brain tissue to expand to its normal dimensions.
Employing advanced high-resolution microscopy techniques, the scientific team meticulously documented the precise manner in which apical progenitor cells underwent division. Under typical, healthy developmental conditions, these specialized cells predominantly divide in an orientation perpendicular to the ventricular surface. This specific mode of division is crucial for ensuring an equitable distribution of cellular components to daughter cells and for generating two new apical progenitor cells, thereby maintaining a robust population for sustained cortical development.
However, within the brain organoids that carried the identified actin mutation, this cardinal division pattern underwent a dramatic alteration. The incidence of vertical divisions, which are critical for self-renewal, was drastically reduced. Instead, horizontal and oblique division angles became the predominant modes of cell replication. The consequence of this skewed orientation was a significantly reduced capacity for apical progenitor cells to replenish themselves. They were more prone to detaching from the ventricular zone, and consequently, were more likely to differentiate into basal progenitor cells rather than continuing their role as self-renewing apical progenitors. "Our detailed analyses provide unequivocal evidence that a fundamental alteration in the orientation of progenitor cell division acts as the decisive catalyst for the observed reduction in brain size," asserted Michael Heide, a group leader at the German Primate Center and the senior author of the study. He further emphasized, "Even a singular alteration within the cellular cytoskeleton is demonstrably sufficient to derail the intricate course of early brain development."
Beyond the alterations in cell division, electron microscopy further revealed subtle, yet significant, structural anomalies at the ventricular surface. The shapes of the cells appeared irregular, and the formation of extraneous protrusions between adjacent cells was observed. Moreover, the researchers detected unusually elevated concentrations of tubulin, another vital cytoskeletal protein integral to the process of cell division, at the junctions between these cells. While the overall structural integrity of the individual cells appeared to remain largely intact, these minute structural abnormalities are hypothesized to exert a lasting influence, permanently altering how cells orient themselves during the critical process of division.
To definitively establish the causal link between the observed developmental defects and the specific actin mutation, thereby excluding the possibility of other genetic variations being responsible, the researchers conducted a critical control experiment. Utilizing the precise gene-editing technology CRISPR/Cas9, they intentionally introduced the identical actin mutation into a healthy line of stem cells. The resultant brain organoids, cultivated from these gene-edited cells, exhibited the same spectrum of developmental defects previously observed in the patient-derived organoids. This experimental outcome served as irrefutable proof that the mutation itself is the primary driving force behind the observed microcephaly.
This significant discovery offers invaluable insights into the intricate pathways through which rare genetic mutations can precipitate complex malformations of the brain. Furthermore, it underscores the immense utility of brain organoids as a powerful tool in contemporary biomedical research. "Our findings significantly enhance our comprehension of how rare genetic disorders can manifest as complex brain malformations and powerfully illustrate the transformative potential of brain organoids in advancing biomedical science," commented Michael Heide.
Nataliya Di Donato, Director of the Institute of Human Genetics at Hannover Medical School, highlighted the clinical implications of the research, stating, "The therapeutic potential stemming from this study is primarily situated within the realm of diagnostics. Our data provides crucial support for the more accurate classification of genetic findings in patients. Given that the pathological processes involved impact early fetal development, direct therapeutic interventions in humans present considerable challenges. Nevertheless, the development of novel pharmacological agents designed to modulate the intricate interactions between actin and microtubules could, in the long term, pave the way for entirely new therapeutic avenues."






