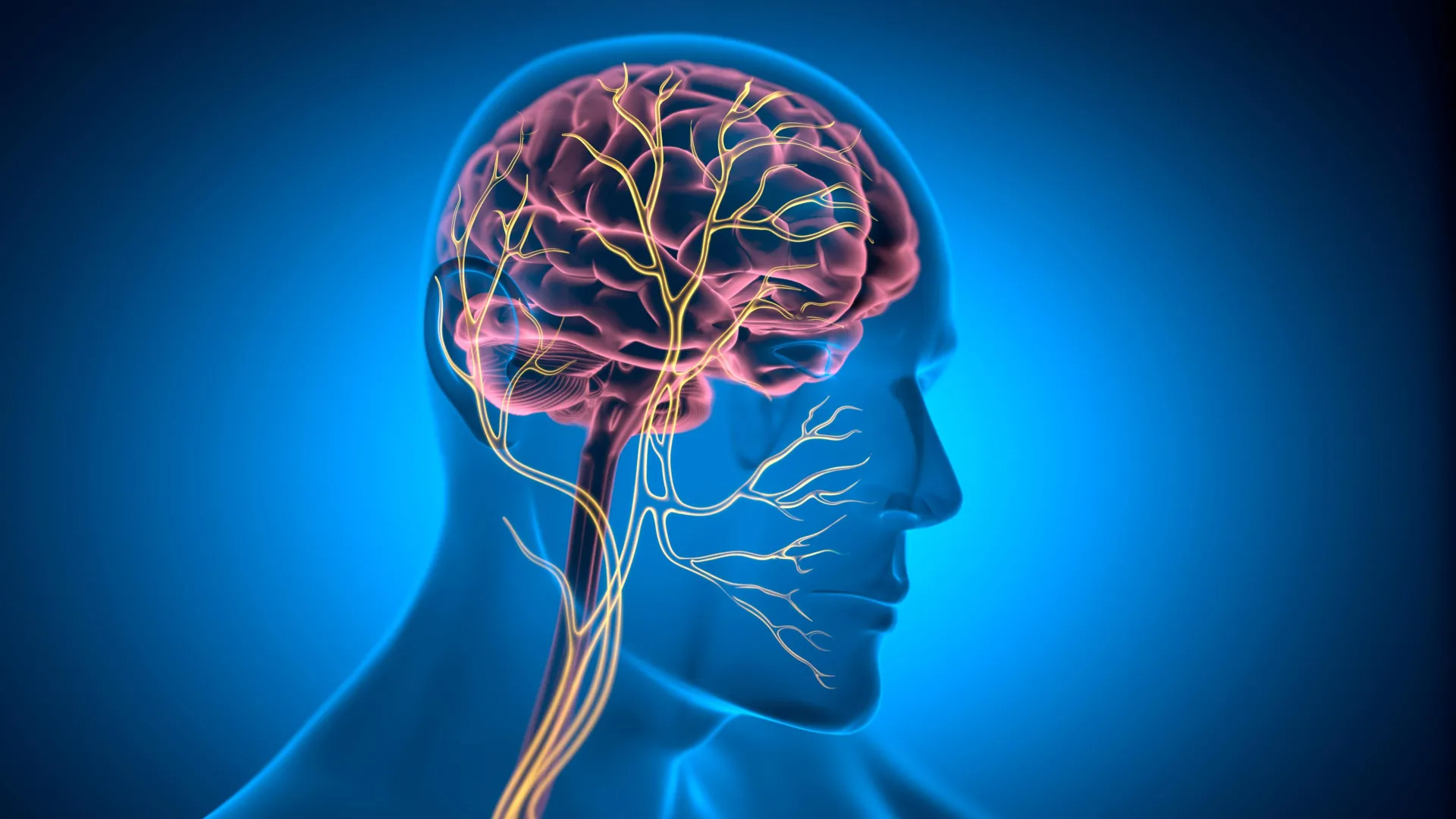
A significant advancement in understanding cardiovascular aging has emerged from a collaborative study, meticulously coordinated by the Sant’Anna School of Advanced Studies in Pisa, and disseminated through the prestigious journal Science Translational Medicine. This pioneering research posits that the vagus nerve, a long cranial nerve extending from the brainstem to the abdomen, holds a pivotal position in maintaining a youthful and robust heart. Specifically, the study elucidates that the preservation of vagal nerve connections to the heart, particularly on both sides, plays an instrumental role in retarding the natural aging process of cardiac tissue. The investigation places particular emphasis on the right cardiac vagus nerve, demonstrating its critical function in safeguarding myocardial cells and fostering enduring cardiac health, irrespective of fluctuations in heart rate.
This intricate investigation was a testament to interdisciplinary synergy, seamlessly integrating experimental medicine with sophisticated bioengineering techniques tailored for cardiovascular research. The project was spearheaded by the Translational Critical Care Unit (TrancriLab) within the Interdisciplinary Research Center Health Science, under the esteemed leadership of Professor Vincenzo Lionetti. A vital contribution to the study’s success came from the Biorobotics Institute, directed by Professor Silvestro Micera, which was instrumental in developing a novel bioabsorbable nerve conduit designed to facilitate the regeneration of the vagus nerve.
The entirety of the experimental endeavors was conducted in Pisa, benefiting from substantial funding secured through the European FET (Future and Emerging Technologies) program via the NeuHeart project. Additional financial support was garnered from PNRR funds allocated by the Tuscany Health Ecosystem, underscoring a commitment to cutting-edge scientific inquiry. The collaborative nature of this research extended to a broad consortium of distinguished academic and research institutions, both within Italy and internationally. This network included the Scuola Normale Superiore, the University of Pisa, the Fondazione Toscana G. Monasterio, the Institute of Clinical Physiology of the National Research Council (CNR), the University of Udine, GVM Care & Research, Al-Farabi Kazakh National University, the Leibniz Institute on Ageing in Jena, and the École Polytechnique Fédérale de Lausanne, collectively pooling expertise and resources.
The significance of maintaining an intact connection with the vagus nerve cannot be overstated, as highlighted by Professor Lionetti. He explains that the integrity of the vagal nerve’s link to the heart is directly correlated with its aging trajectory. When this vital connection is compromised or severed, the heart’s aging process accelerates, leading to a decline in its functional capacity over time. This breakdown in communication can precipitate a cascade of detrimental changes within the cardiac muscle, rendering it more susceptible to age-related ailments.
Intriguingly, the research team discovered that complete restoration of nerve function is not a prerequisite for observing beneficial outcomes. Anar Dushpanova, a cardiologist at TrancriLab and a co-author of the study, elaborated that even a partial re-establishment of the connection between the right vagus nerve and the heart is demonstrably sufficient to counteract the pathological mechanisms of cardiac remodeling. This partial regeneration can effectively preserve the heart’s contractile efficiency, preventing or mitigating the structural and functional deterioration often associated with aging or injury. This finding opens up new avenues for therapeutic interventions, suggesting that even limited nerve recovery could yield significant clinical advantages.
The crucial role of bioengineering in unlocking these profound insights cannot be overlooked. Eugenio Redolfi Riva, a co-inventor of the patent for the neuroprosthesis developed at the Biorobotics Institute, detailed the innovative approach. "We have developed an implantable bioabsorbable nerve conduit designed to promote and guide the spontaneous regeneration of the thoracic vagus nerve at the cardiac level," he stated. This sophisticated device acts as a scaffold, providing a conducive environment for nerve fibers to regrow and re-establish their connections. The bioabsorbable nature of the conduit ensures that it gradually dissolves within the body once its regenerative task is complete, eliminating the need for subsequent removal and minimizing potential complications. This bioengineering marvel represents a significant leap forward in regenerative medicine, offering a tangible solution for nerve repair.
The implications of these findings are far-reaching, particularly for the fields of cardiothoracic surgery and organ transplantation. Professor Lionetti concluded by emphasizing the transformative potential of this research. "Taken together, these results open new perspectives for cardiothoracic and transplant surgery, suggesting that restoring cardiac vagal innervation at the time of surgery may represent an innovative strategy for long-term heart protection, shifting the clinical paradigm from managing late complications associated with premature cardiac aging to their prevention," he articulated. This paradigm shift signifies a proactive approach to cardiac care, moving beyond the management of established conditions to the prevention of their root causes. By incorporating strategies to restore vagal nerve function during surgical procedures, clinicians could potentially safeguard the heart against the accelerated aging that often follows cardiac interventions or transplantation, thereby enhancing patient outcomes and longevity. This novel approach holds the promise of revolutionizing how we approach heart health in the future.