Alzheimer’s disease, a relentless neurodegenerative condition and the foremost global cause of dementia, presents an escalating public health crisis, prompting intensive scientific inquiry into its underlying mechanisms and potential therapeutic interventions. Recent investigations, published in the esteemed journal Aging-US, have illuminated a compelling hypothesis: circulating components within the bloodstream may exert a significant influence on the pace at which Alzheimer’s pathology advances. Through meticulous experimentation involving laboratory mice, researchers observed that exposure to blood derived from older animals appeared to accelerate Alzheimer’s-associated neuropathological changes, conversely, blood originating from younger donors demonstrated a discernible protective effect.
This groundbreaking research was spearheaded by a consortium of esteemed institutions, including the Instituto Latinoamericano de Salud Cerebral (BrainLat) at Universidad Adolfo Ibáñez, in collaboration with the MELISA Institute, the University of Texas Health Science Center at Houston, and Universidad Mayor. The study sought to unravel the intricate interplay between systemic physiological states and central nervous system health, particularly in the context of Alzheimer’s disease.
At its core, Alzheimer’s disease is characterized by the pathological accumulation of beta-amyloid protein aggregates within the brain parenchyma. These aberrant protein formations, known as amyloid plaques, disrupt the critical communication pathways between neurons, initiating a cascade of cellular damage that progressively erodes brain tissue. While beta-amyloid is intrinsically produced within the brain, emerging scientific consensus has indicated its presence in the systemic circulation. This pivotal discovery has ignited a critical line of inquiry: could factors present in the blood contribute to or modulate the progression of Alzheimer’s pathology?
To rigorously address this question, the research team employed Tg2576 transgenic mice, a well-established and widely utilized animal model for studying Alzheimer’s disease due to their propensity to develop amyloid pathology mirroring human disease. Over an extended period of 30 weeks, these mice were subjected to weekly intravenous infusions of blood sourced from either young or aged donor mice. The primary objective of this experimental design was to ascertain whether specific blood-borne constituents could exert an influence on the extent of amyloid plaque deposition in the brain, as well as to assess any resultant impact on cognitive function and observable behaviors.
Dr. Claudia Durán-Aniotz, a leading investigator from the Instituto Latinoamericano de Salud Cerebral (BrainLat) at Universidad Adolfo Ibáñez, underscored the profound implications of these findings, emphasizing the necessity of adopting a holistic perspective that extends beyond the confines of the brain. "This collaborative endeavor, involving a diverse array of academic and research organizations, powerfully reaffirms the critical importance of understanding how systemic factors shape the brain’s microenvironment and directly influence the pathological processes that drive disease progression," she articulated. "By definitively demonstrating that peripheral signals originating from aged blood can modulate central nervous system mechanisms underlying Alzheimer’s pathophysiology, these results unlock novel avenues for exploring therapeutic targets that engage the blood-brain axis."
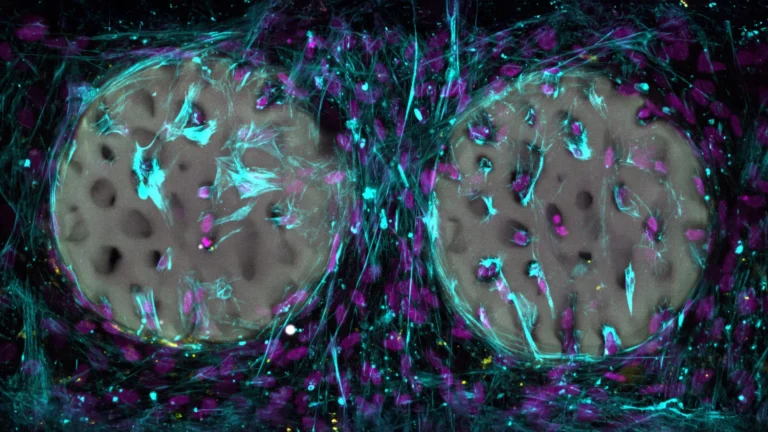
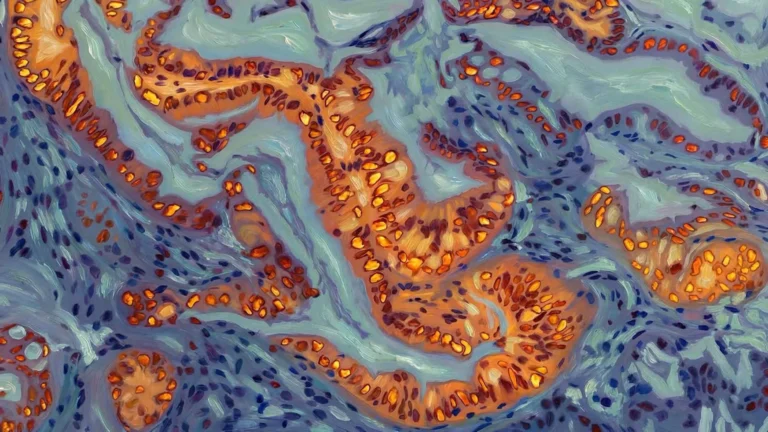
The research methodology incorporated a multifaceted evaluation of cognitive function, utilizing the Barnes maze, a standard behavioral paradigm designed to assess spatial learning and memory. Concurrently, the extent of amyloid plaque accumulation was quantified through sophisticated histological staining techniques and precise biochemical assays. Further deepening the understanding of molecular changes, the scientists conducted an in-depth proteomic analysis of brain tissue harvested from the treated mice. This comprehensive analysis revealed alterations in the activity levels of over 250 distinct proteins. A significant proportion of these identified proteins are intrinsically involved in critical neuronal processes, including synaptic plasticity, endocannabinoid signaling pathways, and calcium channel regulation, thereby offering plausible molecular explanations for the observed disparities in brain health and behavioral outcomes between the experimental groups.
The MELISA Institute played an instrumental role in navigating the complexities of the proteomic data analysis, a technically demanding undertaking. Mauricio Hernández, a specialist in proteomics at the institute, detailed the significant challenges encountered. "Within the scope of this study, we executed a large-scale proteomic analysis that yielded exceptionally high-quality data from a notoriously complex biological matrix such as plasma, which presents a formidable technical hurdle for any proteomics laboratory," he stated. "Leveraging our cutting-edge instrumentation, specifically the timsTOF Pro2 platform, we are exceedingly proud to have contributed to the generation of a robust and scientifically rigorous publication."
These findings contribute significantly to a growing body of evidence suggesting that factors circulating within the blood can exert a direct and measurable impact on the trajectory of neurodegenerative disorders, including Alzheimer’s disease. By precisely identifying the specific blood-borne signals that influence brain pathology, the scientific community may be poised to uncover novel therapeutic targets and devise innovative strategies aimed at decelerating or potentially preventing disease onset and progression. Future research endeavors will be critically focused on pinpointing the exact molecular entities responsible for these observed effects and meticulously evaluating their potential for safe and effective therapeutic application in human patients.
"It is a profound privilege to contribute our proteomic expertise in support of pioneering research initiatives such as this study, which facilitates the advancement of knowledge and the development of novel therapeutic modalities for neurodegenerative diseases, conditions that currently represent a formidable global health challenge," commented Dr. Elard Koch, Chairman of the MELISA Institute.
This ambitious research program received substantial financial and institutional support from a variety of sources, reflecting the global scientific community’s commitment to addressing Alzheimer’s disease. Dr. C.D.A. was supported by grants from ANID/FONDECYT Regular 1210622 and ANID/PIA/ANILLOS ACT210096, alongside funding from the Alzheimer’s Association (AARGD-24-1310017), ANID/FOVI240065, and ANID/Proyecto Exploracion 13240170. Further crucial support was provided by the MULTI-PARTNER CONSORTIUM TO EXPAND DEMENTIA RESEARCH IN LATIN AMERICA (ReDLat), funded by NIH research grants R01AG057234, administered by the National Institute on Aging (NIA) and the Fogarty International Center (FIC). Additional funding was secured through an Alzheimer’s Association grant (SG-20-725707-ReDLat), the Rainwater Charitable Foundation, and the Global Brain Health Institute. Further complementary support was provided by the Bluefield Project to Cure Frontotemporal Dementia, an NIH contract (75NS95022C00031), and the NIA under awards R01AG075775, R01AG082056, and R01AG083799. The content presented herein is solely the responsibility of the authors and does not necessarily reflect the official viewpoints of the National Institutes of Health, the Alzheimer’s Association, the Rainwater Charitable Foundation, the Bluefield Project to Cure Frontotemporal Dementia, or the Global Brain Health Institute. The contributions of Dr. R.M. and his team to this research were bolstered by NIH grants RF1AG072491 and RF1AG059321. Dr. U.W.’s participation was supported by ANID/FONDECYT Regular 1240176.