A groundbreaking scientific endeavor in Japan has achieved a significant milestone in neuroscience by successfully cultivating intricate, multi-region human brain circuits within a laboratory setting, offering unprecedented insights into the foundational wiring of the human cerebrum. These sophisticated constructs, dubbed "assembloids," are meticulously engineered from induced pluripotent stem cells (iPSCs) and are designed to faithfully replicate the complex interconnections and communication pathways characteristic of distinct areas within the human brain. Through the application of this innovative system, researchers have definitively demonstrated the pivotal role played by the thalamus in orchestrating the development of specialized neural circuits within the cerebral cortex, a revelation published in the esteemed journal Proceedings of the National Academy of Sciences of the United States of America.
The cerebral cortex, the brain’s outermost layer, is a marvel of biological engineering, housing a vast array of specialized neurons that must engage in precise and efficient communication, not only amongst themselves but also with other brain regions. These intricate connections form the bedrock of essential cognitive functions, encompassing sensory perception, abstract thought, and higher-level reasoning. Understanding the genesis and maturation of these neural circuits is paramount, particularly in the context of neurodevelopmental conditions. Disorders such as autism spectrum disorder (ASD), for instance, are often characterized by aberrant development or dysfunctional operation of these cortical circuits. Consequently, unraveling the biological underpinnings of neural circuit formation and maturation is a critical step towards identifying the root causes of these complex disorders and formulating effective therapeutic interventions.
Historically, research in animal models, particularly rodents, has suggested a significant role for the thalamus in the organizational processes of cortical neural circuits. However, the precise nature of the reciprocal interactions between the thalamus and the cortex during the intricate dance of circuit formation within the human brain has remained largely enigmatic. Direct investigation of these processes in living humans is fraught with ethical and technical impediments, primarily due to the inherent difficulties in obtaining viable human brain tissue for study. To surmount these formidable obstacles, the scientific community has increasingly turned to the development of organoids – three-dimensional cellular structures cultivated from stem cells that bear a remarkable resemblance to actual organs, including the brain.
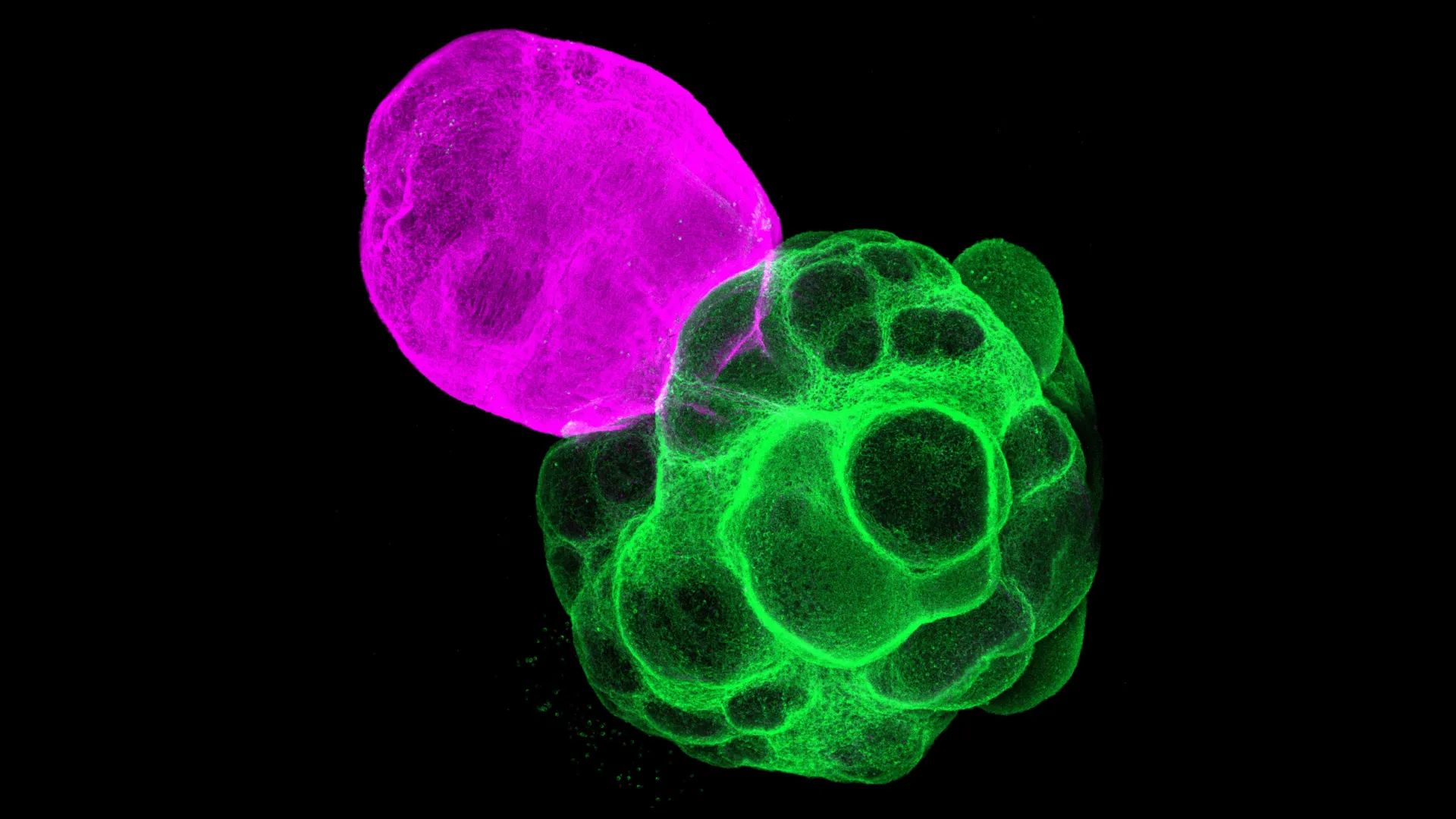
While brain organoids have proven invaluable for studying the development of individual brain regions, they possess a fundamental limitation: a single organoid cannot adequately capture the dynamic and complex interplay that occurs between disparate brain areas. To address this deficiency and enable a more realistic examination of neural circuit formation, researchers have advanced to the utilization of "assembloids." These sophisticated structures are created by the physical aggregation and fusion of two or more distinct organoids, thereby simulating the anatomical and functional relationships found in the intact brain.
In this pioneering study, Professor Fumitaka Osakada, alongside graduate student Masatoshi Nishimura and their dedicated colleagues at the Graduate School of Pharmaceutical Sciences at Nagoya University, meticulously engineered assembloids specifically designed to model the crucial interactions between the thalamus and the cerebral cortex. The research team commenced by independently generating separate cortical and thalamic organoids, both derived from human iPSCs. Subsequently, these meticulously cultured organoids were carefully fused together, creating a novel experimental system that allowed the scientists to meticulously observe and analyze the emergent interactions between these two vital brain regions as they progressed through developmental stages.
The experimental observations yielded compelling evidence of robust and directed axonal growth between the fused organoids. Nerve fibers originating from the thalamic organoid were observed to extend significantly towards the cortical organoid, while concurrently, cortical nerve fibers demonstrated a reciprocal growth pattern, projecting towards the thalamus. Crucially, these axonal projections established synaptic connections with each other, forming functional junctions that closely mirrored the intricate wiring patterns observed in the developing human brain.
To quantitatively assess the impact of this inter-regional communication on developmental trajectories, the researchers conducted a comparative analysis of gene expression profiles within the cortical tissue of the assembloid and that of a standalone cortical organoid. The findings revealed a distinct signature of enhanced maturity in the cortical tissue that was in direct communication with the thalamus. This observation strongly suggests that the bidirectional signaling between the thalamus and the cortex acts as a potent stimulus, actively promoting accelerated cortical growth and more advanced developmental maturation.
Further investigations delved into the dynamics of signal propagation within the assembloid, revealing fascinating patterns of neural activity. The scientists observed that neuronal signals propagated from the thalamus into the cortex in coordinated, wave-like sequences, a phenomenon that resulted in the emergence of synchronized electrical activity across the interconnected cortical neural networks. To pinpoint the specific neuronal populations responsible for this synchronized behavior, the research team meticulously measured the electrical activity within three principal categories of excitatory neurons found in the cortex: intratelencephalic (IT) neurons, pyramidal tract (PT) neurons, and corticothalamic (CT) neurons.
The analysis demonstrated that synchronized neural activity was prominently exhibited by PT and CT neurons, both of which are characterized by their projection pathways that send signals back to the thalamus. In contrast, IT neurons, which do not establish direct connections with the thalamus, did not display the same degree of synchronized activity. This differential response pattern provides critical evidence that thalamic input selectively modulates and strengthens the activity of specific neuronal subtypes, thereby facilitating their integration into coordinated functional networks and promoting their overall functional maturation.
The successful reconstruction of human neural circuits using the assembloid platform represents a transformative advancement, establishing a powerful and versatile new experimental paradigm for investigating the multifaceted processes of brain circuit formation, functional operation, and the diverse cellular and regional variations that characterize the human brain. Professor Osakada articulated the profound significance of this research, stating, "We have achieved substantial progress in the constructivist approach to comprehending the human brain through its faithful reproduction. We are confident that these discoveries will serve to accelerate the elucidation of the underlying mechanisms of neurological and psychiatric disorders, while simultaneously fostering the development of novel therapeutic strategies." This innovative methodology holds immense promise for future research endeavors, paving the way for deeper understanding and more effective treatments for a wide spectrum of brain-related conditions.






