A groundbreaking advancement in oncology, spearheaded by researchers at the Korea Advanced Institute of Science and Technology (KAIST), offers a novel strategy to reprogram the body’s own immune cells directly within solid tumors, effectively converting them into potent anti-cancer agents. This innovative method circumvents many of the logistical and biological hurdles that have historically limited the efficacy and accessibility of cell-based immunotherapies, particularly for challenging solid malignancies. The core of this breakthrough lies in the targeted delivery of genetic instructions and immune-boosting compounds to macrophages already present within the tumor microenvironment, transforming these often-suppressed cells into active cancer destroyers.
Solid tumors, encompassing prevalent and aggressive cancers such as those of the stomach, lung, and liver, represent a formidable challenge in modern medicine. Their dense, complex architecture creates physical barriers that prevent immune cells from infiltrating and effectively engaging cancerous cells. Beyond physical impedance, the tumor microenvironment (TME) is notoriously immunosuppressive, actively hijacking and disarming immune cells that do manage to penetrate its defenses. Among these co-opted immune cells are tumor-associated macrophages (TAMs), which, despite their inherent potential to phagocytose and eliminate pathogens and abnormal cells, are often skewed by the TME into a pro-tumorigenic state. In this altered state, TAMs can inadvertently promote tumor growth, angiogenesis, and metastasis, rather than fulfilling their natural anti-cancer roles. This hostile internal landscape significantly compromises the effectiveness of many existing immunotherapies, which frequently struggle to overcome these multifaceted biological and physical obstacles.
Chimeric Antigen Receptor (CAR) therapies have revolutionized the treatment of certain hematological cancers, demonstrating remarkable success by genetically engineering a patient’s T-cells to recognize and attack cancer cells. However, applying CAR-T cell therapy to solid tumors has proven difficult due to the aforementioned TME barriers and the inherent limitations of T-cells in navigating and persisting within these dense structures. Recognizing the unique advantages of macrophages, which are naturally equipped to infiltrate solid tissues and engulf large particles, the scientific community has increasingly turned its attention to CAR-macrophage (CAR-M) therapy as a promising next-generation approach. Macrophages possess an intrinsic ability not only to directly consume cancer cells but also to present antigens and secrete cytokines, thereby stimulating a broader immune response.
Despite their significant promise, existing CAR-macrophage therapies face substantial practical challenges. Current methodologies typically involve an elaborate multi-step process: immune cells are first extracted from a patient’s blood, meticulously processed and genetically modified in a specialized laboratory setting to express the CAR protein, expanded to therapeutic numbers, and then re-infused into the patient. This intricate ex vivo manipulation is not only time-consuming and labor-intensive but also prohibitively expensive and difficult to scale, thereby limiting its widespread applicability and accessibility for a large patient population. The logistics involved in maintaining sterility, ensuring cell viability, and navigating regulatory pathways add further layers of complexity.
To address these critical limitations, the research team at KAIST, under the leadership of Professor Ji-Ho Park from the Department of Bio and Brain Engineering, conceived and developed an innovative in-situ reprogramming strategy. Their approach ingeniously bypasses the need for ex vivo cell extraction and manipulation by targeting the tumor-associated macrophages that already reside within the cancerous tissue. The core innovation involves a sophisticated delivery system: lipid nanoparticles (LNPs) engineered for efficient uptake by macrophages. These nanoparticles serve as microscopic vehicles, precisely encapsulating two crucial components: messenger RNA (mRNA) encoding the Chimeric Antigen Receptor (CAR) protein, which provides cancer-recognition instructions, and an immune-activating compound designed to enhance the macrophages’ intrinsic anti-tumor functions.
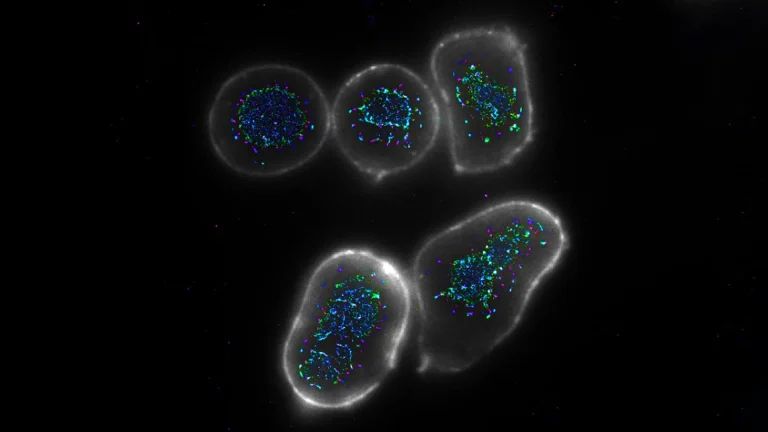
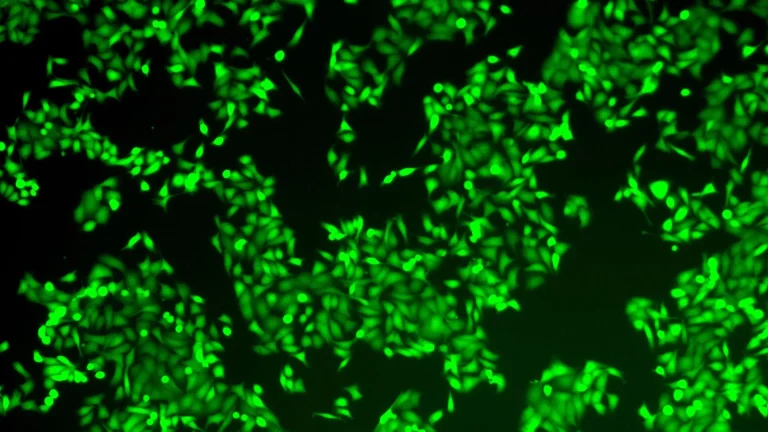
Upon direct injection into the tumor, these specially formulated lipid nanoparticles are readily internalized by the local macrophage population. Once inside the cells, the mRNA payload is translated into CAR proteins, endowing the macrophages with the ability to specifically identify and bind to cancer cells. Simultaneously, the co-delivered immune-activating compound works synergistically to "wake up" and potentiate these newly reprogrammed CAR-macrophages. This dual action ensures that the macrophages not only gain the capacity to target cancer but are also primed to exert a robust effector function, effectively transforming dormant or pro-tumorigenic resident macrophages into highly active and targeted anti-cancer immune cells directly within their native environment. This "direct conversion" within the body represents a paradigm shift from traditional cell therapies.
The efficacy of this novel in-situ CAR-macrophage therapy was rigorously evaluated in preclinical animal models. In studies involving melanoma, an aggressive form of skin cancer, direct intratumoral injection of the LNP-based treatment led to a rapid uptake by macrophages and subsequent production of cancer-recognizing proteins. The concurrent activation of immune signaling pathways resulted in "enhanced CAR-macrophages" exhibiting significantly superior cancer-killing capabilities. These reprogrammed cells were observed to not only directly eliminate tumor cells but also to orchestrate a broader immune response by stimulating surrounding immune cells. A notable finding from these animal studies was the significant reduction in tumor growth observed in treated subjects. Furthermore, researchers uncovered evidence of an abscopal effect, where the immune response extended beyond the directly treated tumor, suggesting the potential for systemic, body-wide immune protection against distant metastases or untreated tumor sites. This phenomenon, where localized treatment generates an immune response capable of shrinking tumors elsewhere in the body, is a highly sought-after outcome in cancer immunotherapy.
Professor Ji-Ho Park emphasized the profound implications of this research, stating that the study introduces a fundamentally new concept for immune cell therapy. He highlighted its significance in simultaneously addressing two critical limitations of existing CAR-macrophage therapies: the challenges associated with efficient delivery of therapeutic cells and the pervasive immunosuppressive environment within solid tumors. By generating anti-cancer immune cells directly inside the patient’s body, the method streamlines the therapeutic process, potentially reducing costs and improving accessibility. This endogenous generation of therapeutic cells within the TME itself offers a unique advantage, as these cells are inherently adapted to their environment and can more effectively navigate and persist within the tumor.
The successful development of this innovative approach could pave the way for more effective and widely applicable treatments for a broad spectrum of solid tumors that currently resist conventional immunotherapies. Future research will likely focus on optimizing the LNP delivery system, exploring different CAR targets, and investigating combination strategies with other anti-cancer agents. Clinical translation will involve comprehensive safety and efficacy trials in human subjects, a critical step toward realizing the full therapeutic potential of this in-situ cellular engineering strategy.
This significant research was primarily conducted by Jun-Hee Han, Ph.D., from the Department of Bio and Brain Engineering at KAIST, serving as the first author of the study. The comprehensive findings were formally published on November 18 in ACS Nano, a highly respected international scientific journal dedicated to advancements in nanotechnology. Financial backing for this pioneering work was generously provided by the Mid-Career Researcher Program, administered by the National Research Foundation of Korea, underscoring the national commitment to fostering cutting-edge scientific innovation in medical research.