Recent scientific inquiry emanating from Japan’s Nagoya University has illuminated a previously unrecognized hazard associated with prevalent ophthalmic preparations, specifically those formulated with a petrolatum base. This groundbreaking research reveals that such ointments can induce significant swelling, and in certain instances, lead to the structural failure of a widely deployed glaucoma implant device. Through a rigorous combination of real-world patient observations and controlled laboratory investigations, scientists have definitively demonstrated that these topical treatments can compromise the integrity of the PRESERFLO MicroShunt, a surgical implant utilized in the management of glaucoma across more than sixty nations.
This pioneering study stands as the inaugural effort to integrate clinical case evidence with empirical data, establishing a clear causal link between the application of petrolatum-based eye ointments and adverse structural alterations within this specific type of intraocular device. Glaucoma, a progressive ocular condition characterized by damage to the optic nerve, represents a significant global health challenge, potentially culminating in irreversible visual impairment. The underlying pathology is frequently attributed to elevated intraocular pressure, a consequence of compromised fluid drainage within the eye. Epidemiological estimates suggest that glaucoma impacts a substantial segment of the global population, numbering in the tens of millions.
For individuals grappling with glaucoma, surgical intervention offers a critical avenue for treatment, and the MicroShunt has emerged as a significant advancement in this domain. This miniaturized filtration device is surgically introduced into the eye to facilitate the more efficient egress of excess aqueous humor, thereby mitigating intraocular pressure. When contrasted with more traditional glaucoma surgical procedures, the MicroShunt offers the distinct advantage of a reduced incidence of postoperative complications and frequently diminishes the reliance on ongoing pharmacological interventions.
The inherent vulnerability of the implant material to specific chemical interactions is central to understanding this newly identified risk. The PRESERFLO MicroShunt is meticulously engineered from a styrenic thermoplastic elastomer, a sophisticated polymer composition known as polystyrene-block-polyisobutylene-block-polystyrene (SIBS). This advanced material was selected for its inherent flexibility, exceptional biocompatibility, and its propensity to minimize inflammatory responses and the development of intraocular scarring, factors crucial for long-term ocular health and implant function.
However, the very characteristics that render the SIBS material beneficial also render it susceptible to degradation upon contact with hydrocarbon- and oil-based substances. Its pronounced affinity for lipids means that petrolatum-based eye ointments can readily permeate the implant’s structure. Upon ingress into the polymer matrix, these oily components can instigate a process of expansion, leading to undesirable alterations in the device’s dimensions and its mechanical resilience.
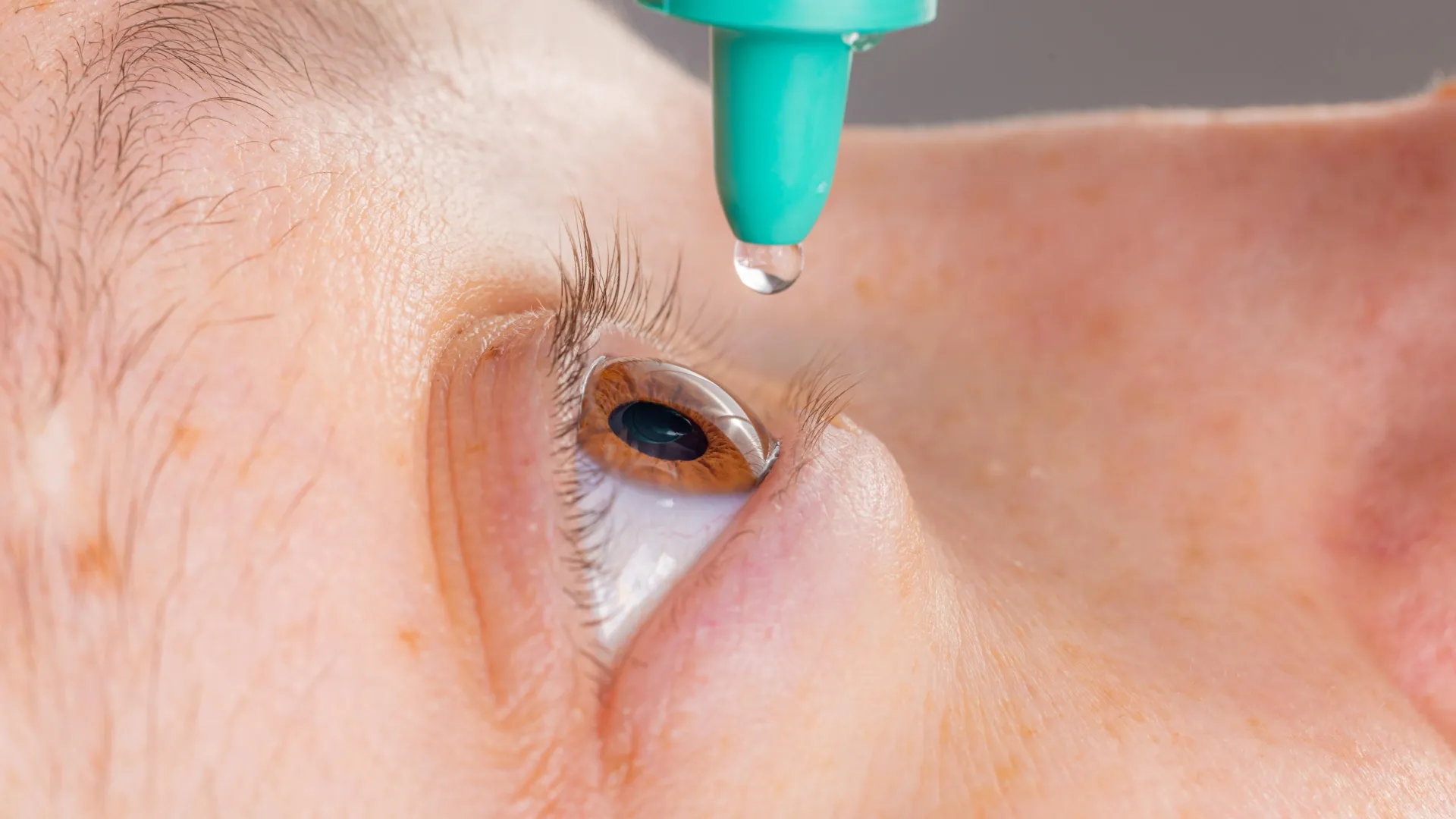
Crucially, the manufacturer of the MicroShunt includes explicit directives warning against such potential exposures. The product’s accompanying instructions clearly state that "the MicroShunt should not be subjected to direct contact with petrolatum-based (i.e., petrolatum jelly) materials, such as ointments and dispersions." Despite this explicit guidance, the severity of this warning appears not to be universally recognized or consistently adhered to within the clinical milieu.
Assistant Professor Ryo Tomita of the Nagoya University Graduate School of Medicine, the lead author of the study, observed firsthand the consequences of this interaction. "Swollen MicroShunts can be structurally fragile," he stated, recounting an instance where he witnessed a rupture in a swollen implant during a surgical procedure. Professor Tomita emphasized that enhanced awareness among clinicians regarding this specific risk could significantly contribute to the prevention of similar adverse events.
The comprehensive investigation into this phenomenon was a testament to interdisciplinary collaboration, uniting expertise from both the medical and engineering fields. Professor Tomita collaborated closely with Assistant Professor Taiga Inooka and Associate Professor Kenya Yuki from Nagoya University Hospital and the Graduate School of Medicine. Their efforts were augmented by the contributions of Dr. Takato Kajita and Junior Associate Professor Atsushi Noro from the Graduate School of Engineering, who brought their specialized knowledge of material science to bear on the problem. Together, this multidisciplinary team embarked on a mission to meticulously document how the MicroShunt’s physical characteristics transform following exposure to petrolatum-based eye ointments.
While the medical contingent of the research team focused on analyzing retrospective patient data, the engineering researchers undertook a series of meticulously designed laboratory experiments to replicate and elucidate the observed effects. The findings of this collaborative endeavor were subsequently published in the esteemed peer-reviewed journal, Graefe’s Archive for Clinical and Experimental Ophthalmology, lending significant weight and credibility to their conclusions.
The clinical segment of the investigation involved a detailed examination of seven glaucoma patients whose PRESERFLO MicroShunt implants had been surgically removed for a variety of clinical indications. A striking and consistent pattern emerged from this cohort, directly correlating implant integrity with the history of exposure to petrolatum-based ointments. In three distinct cases where the MicroShunt was found to be exposed externally, beyond the protective conjunctival tissue, and the patients had received treatment with a petrolatum-based eye ointment, all three devices exhibited discernible swelling. More alarmingly, two of these swollen implants had sustained ruptures, indicating a catastrophic failure of their structural integrity.
Conversely, in another group of three patients, the MicroShunt remained fully encapsulated by the conjunctiva, and no petrolatum-based ointments were administered. These implants, in stark contrast, retained their original anatomical form and structural characteristics, providing compelling evidence of the protective role of the conjunctiva in preventing direct contact.
A particularly illuminating case within the study further underscored the direct causal relationship. In this instance, the MicroShunt was exposed externally, akin to the patients who experienced swelling, yet crucially, no petrolatum-based ointment was applied. This particular implant remained unaffected, demonstrating that conjunctival exposure alone was not the precipitating factor for swelling; rather, direct chemical contact with the ointment was the primary culprit.
The laboratory experiments served to unequivocally validate the clinical observations and provide a mechanistic explanation for the observed phenomena. Researchers meticulously immersed explanted, unused MicroShunts in a standardized petrolatum-based eye ointment, thereby recreating the conditions that mimicked the changes observed in the patient population.
Subsequent microscopic analysis revealed a rapid and significant expansion of the implant material. After a mere 24-hour period of immersion, the external diameter of the MicroShunt had increased by a factor of 1.44 compared to its original dimensions. Furthermore, the delicate fin-like projections, essential components for the implant’s function, widened by an average of 1.29 times their initial size.
The underlying cause of this dramatic volumetric increase was further elucidated through detailed chemical analysis. Within the initial 24 hours of submersion, the oil components absorbed from the ointment constituted approximately 45% of the MicroShunt’s total mass. This infiltration continued over time, with the oil content escalating to an impressive 73% after three months of continuous exposure. These quantitative findings conclusively demonstrated that the observed swelling was a direct consequence of the preferential absorption of lipophilic components from the oil-based ointment into the polymer matrix of the implant.
The implications of these findings for the clinical management of glaucoma patients who have received MicroShunt implants are profound. The research team strongly advises ophthalmologists to refrain from prescribing or applying petrolatum-based eye ointments to patients with these devices, particularly in situations where the implant is exposed externally. They advocate for the diligent consideration of alternative post-operative therapeutic agents. Furthermore, the study highlights the necessity for ongoing research to ascertain whether subtle, sub-rupture swelling might still exert a detrimental impact on the long-term efficacy and performance of the MicroShunt implant, even in the absence of overt structural failure.
Reflecting on the broader significance of their work, Associate Professor Noro emphasized the critical need for a comprehensive understanding of medical materials. "Our study found that commonly used medical materials can cause unexpected complications if their chemical properties and usage environments are not fully understood," he stated. He further underscored the imperative, from both medical and engineering perspectives, to prioritize a deep comprehension of the chemical characteristics of medical materials and to implement judicious management strategies for their application and environmental context.