A pioneering advancement in the fusion of neuroscience and bioelectronic engineering has emerged from Northwestern University, where researchers have successfully developed a novel wireless implant capable of delivering information directly into the brain via patterned light. This sophisticated technology circumvents the body’s conventional sensory pathways, instead interfacing with neural circuits by stimulating neurons with precisely controlled photonic emissions. The implant’s design prioritizes minimal invasiveness and seamless integration, representing a significant leap in our ability to communicate with the central nervous system.
The device itself is characterized by its remarkable flexibility and bio-compatible softness, allowing it to rest comfortably against the skull beneath the scalp. From this unobtrusive position, it meticulously projects carefully orchestrated sequences of light through the cranial bone, effectively activating specific neuronal populations distributed across the cerebral cortex. This novel method bypasses the need for surgical penetration of the brain tissue, a common limitation in many existing neuro-interfacing technologies.
During rigorous experimental trials conducted on rodent models, scientists employed minuscule, meticulously timed pulses of light to selectively engage targeted groups of neurons situated deep within the brain. Crucially, these neurons had been genetically engineered to exhibit photic sensitivity, meaning they respond specifically to light stimulation. The study revealed that the animal subjects, presented with these artificial light cues, demonstrated a remarkable capacity for rapid learning and adaptation. Even in the absence of traditional sensory inputs such as sound, sight, or physical touch, the mice were able to interpret the incoming photonic information, utilizing it to make informed decisions and successfully complete assigned behavioral tasks with impressive accuracy.
The potential implications of this groundbreaking technology are vast and far-reaching, promising to revolutionize a wide spectrum of medical applications. Experts envision its use in restoring sensory feedback for individuals with prosthetic limbs, enabling them to feel a sense of touch or proprioception. Furthermore, the technology could pave the way for advanced artificial sensory prostheses, potentially restoring rudimentary forms of hearing or vision for those who have lost these senses. The ability to precisely control neural activity also opens avenues for enhanced robotic limb control, accelerated rehabilitation processes following strokes or injuries, and the development of novel, non-pharmacological methods for modulating pain perception.
This seminal research, detailing the intricate design and experimental validation of the light-based neural interface, has been formally published in the esteemed scientific journal Nature Neuroscience, with its release scheduled for Monday, December 8th. The publication marks a significant milestone in the field of neurobiology and bioelectronics, offering a detailed account of the methodology and findings that underscore this transformative development.
"Our brains are perpetually engaged in the complex process of translating electrical activity into conscious experiences, and this innovative technology provides us with an unprecedented means to directly engage with that fundamental mechanism," explained Yevgenia Kozorovitskiy, a distinguished neurobiologist at Northwestern University who spearheaded the experimental facets of the investigation. "This versatile platform empowers us to generate entirely novel neural signals and subsequently observe how the brain adapts and learns to interpret and utilize them. It represents a crucial step towards the ultimate goal of restoring lost sensory capabilities following injury or disease, while simultaneously offering profound insights into the foundational principles that govern our perception of the world."
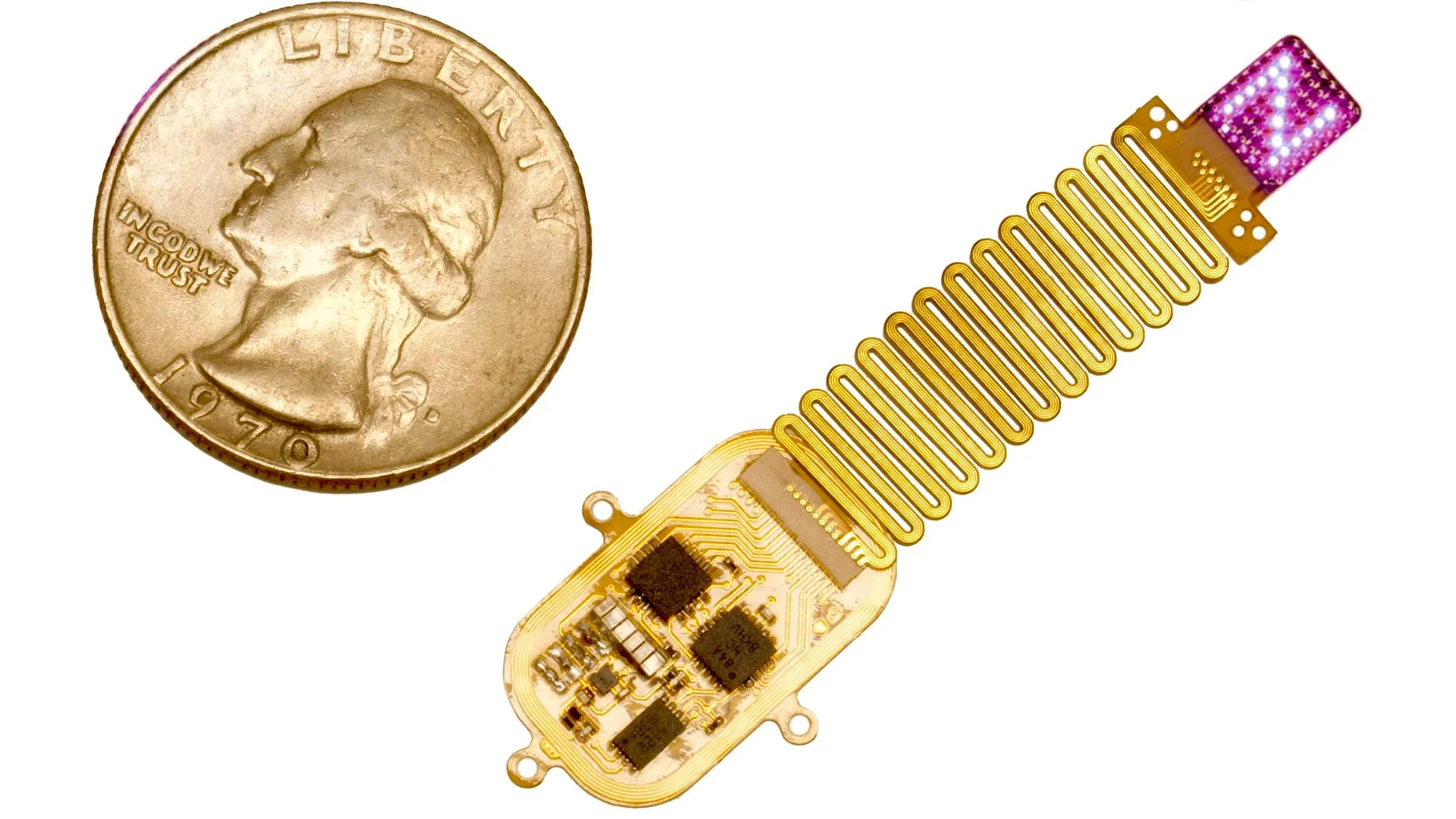
John A. Rogers, a globally recognized authority in bioelectronics and the principal investigator overseeing the technological development of the implant, elaborated on the engineering challenges and achievements. "The creation of this device necessitated a fundamental re-evaluation of how to deliver patterned stimulation to the brain in a manner that is both minimally invasive and fully implantable," Rogers stated. "By ingeniously integrating a supple, conformable array of micro-light-emitting diodes (micro-LEDs)—each astonishingly minute, comparable in size to a single strand of human hair—with a wirelessly powered control module, we have engineered a system that can be programmed in real-time. Critically, this system operates entirely beneath the skin, without eliciting any discernible adverse effects on the natural behaviors of the test subjects. This represents a monumental stride in the development of devices that can interface with the brain without the encumbrance of cumbersome wires or bulky external hardware. Its utility is twofold: immediate benefits for fundamental neuroscience research and significant long-term potential for addressing critical health challenges in human populations."
Kozorovitskiy holds the esteemed Irving M. Klotz Professorship of Neurobiology within Northwestern’s Weinberg College of Arts and Sciences and is an integral member of the Chemistry of Life Processes Institute. Rogers, a multifaceted scholar, holds appointments in materials science and engineering, biomedical engineering, and neurological surgery, and serves as the director of the Querrey Simpson Institute for Bioelectronics. The lead author of this groundbreaking study is Mingzheng Wu, a dedicated postdoctoral researcher whose contributions were pivotal to the project’s success.
This latest research represents a significant evolution from the team’s earlier breakthroughs in optogenetics, a field that utilizes light to control genetically modified neurons. In a notable prior achievement in 2021, the same group unveiled the first fully implantable, programmable, wireless, and battery-free device capable of controlling neuronal activity with light. That earlier system, employing a single micro-LED probe, successfully influenced social behaviors in mice. A key advantage over traditional optogenetic techniques, which often required restrictive fiberoptic wires, was the wireless design, which permitted the mice to exhibit natural behaviors in their social environments without impediment.
The newly developed implant significantly expands upon this foundational work, enabling a far more intricate and nuanced form of communication with the brain. Rather than stimulating a single, localized region, the advanced system incorporates an array comprising up to 64 independently controllable micro-LEDs. This sophisticated arrangement allows for the delivery of dynamic sequences of light, meticulously designed to mimic the distributed patterns of neural activity that naturally occur within the brain during sensory experiences. Because genuine sensations engage widespread neural networks rather than isolated neurons, this multi-site stimulation approach more accurately replicates the complex functional architecture of the cerebral cortex.
"In our initial publication, we demonstrated the capability of a single micro-LED," noted Wu. "Our current work utilizes an array of 64 micro-LEDs to precisely orchestrate patterns of cortical activity. The sheer combinatorial possibilities—in terms of frequency, intensity, and temporal sequencing—that can be generated with this array are virtually limitless, offering an unprecedented level of control over neural stimulation."
Despite its enhanced functionality, the device maintains a remarkably compact profile. Measuring approximately the dimensions of a postage stamp and possessing a thickness less than that of a credit card, it represents a triumph of miniaturization. Crucially, the new iteration avoids the need for direct insertion into the brain; instead, its soft, adaptable material conforms gently to the surface of the skull, directing light stimuli through the bone.
"Red light possesses excellent tissue penetration properties," explained Kozorovitskiy. "It is capable of reaching sufficiently deep into the brain to effectively activate neurons through the intact skull."
To rigorously assess the efficacy of the system, the research team collaborated with mice genetically modified to possess light-responsive neurons within their cerebral cortex. The animals underwent a training regimen designed to associate specific patterns of light stimulation with a tangible reward, typically dispensed from a designated port within the experimental chamber. During a series of meticulously designed experiments, the implant delivered a predetermined light pattern across four distinct cortical regions, effectively functioning as a form of coded message transmitted directly into the brain. The mice demonstrated a remarkable ability to discern this target pattern from numerous other stimuli. Upon detecting the correct artificial signal, they would navigate to the corresponding port to receive their reward.
"By consistently selecting the correct port, the animal unequivocally demonstrated that it had successfully received and interpreted the transmitted message," Wu emphasized. "Since they cannot articulate their sensory experiences through language, their behavior serves as our primary means of understanding their perceptions."
With the successful demonstration that the brain can indeed interpret patterned light stimulation as meaningful information, the research team is now poised to explore more complex signal patterns and to quantify the extent of distinct signals the brain can reliably learn and process. Future iterations of the device are anticipated to feature an increased number of micro-LEDs, reduced spacing between them for finer spatial resolution, larger arrays to encompass broader cortical areas, and the utilization of specific light wavelengths with enhanced tissue penetration capabilities.
This groundbreaking study, titled "Patterned wireless transcranial optogenetics generates artificial perception," received substantial support from a consortium of esteemed organizations, including the Querrey Simpson Institute for Bioelectronics, the National Institute of Neurological Disorders and Stroke (NINDS) through the BRAIN Initiative, the National Institute of Mental Health, the One Mind Nick LeDeit Rising Star Research Award, the Kavli Exploration Award, the Shaw Family Pioneer Award, the Simons Foundation, the Alfred P. Sloan Foundation, and the Christina Enroth-Cugell and David Cugell Fellowship. This broad base of support highlights the significant scientific interest and potential impact of this pioneering research.






