A groundbreaking investigation conducted by scientists affiliated with the Institut Pasteur and Inserm has illuminated a potentially far-reaching influence of common dietary emulsifiers on the health trajectory of future generations. The research, meticulously detailed in the esteemed journal Nature Communications, reveals that when female mice consume these ubiquitous food additives, their offspring experience profound modifications to their intestinal microbial communities from the earliest stages of life. These nascent alterations in gut flora are subsequently associated with an elevated susceptibility to chronic inflammatory conditions affecting the digestive tract, as well as an increased likelihood of developing obesity later in adulthood. This discovery underscores a critical need for comprehensive human epidemiological and clinical studies to fully ascertain the intergenerational health implications of early-life exposure to such food components.
Emulsifiers represent a class of substances extensively incorporated into a vast array of industrially processed food products. Their primary function is to stabilize mixtures of ingredients that would otherwise separate, such as oil and water, thereby enhancing product consistency, texture, and extending shelf stability. Consumers encounter these compounds daily in items ranging from creamy dairy alternatives and various baked goods to frozen desserts and even certain formulations of powdered infant nourishment. Despite their pervasive presence in the contemporary diet, the scientific community’s understanding of their full spectrum of biological effects, particularly concerning the delicate balance of the human gastrointestinal microbiome, remains notably limited. Regulatory bodies often grant these additives "Generally Recognized As Safe" (GRAS) status based on historical usage and toxicology assessments, yet evolving scientific methodologies are continually refining our insights into subtle, long-term biological interactions.
The experimental design for this pivotal study was conceived and executed under the direction of Dr. Benoit Chassaing, a distinguished Research Director at Inserm and the principal investigator leading the Microbiome-Host Interactions laboratory, an integral unit within the Institut Pasteur. To rigorously investigate the hypothesis, the research team administered two specific emulsifying agents—carboxymethyl cellulose, frequently identified as E466 in European food labeling, and polysorbate 80, also known as E433—to a cohort of female laboratory mice. This dietary regimen commenced a full ten weeks prior to the onset of pregnancy and was maintained consistently throughout both the gestation period and the subsequent lactation phase. Crucially, the researchers then meticulously analyzed the nascent microbial populations within the digestive systems of the offspring, none of whom had ever directly ingested the emulsifiers themselves. This indirect exposure model was central to understanding the potential for maternal dietary components to shape offspring biology.
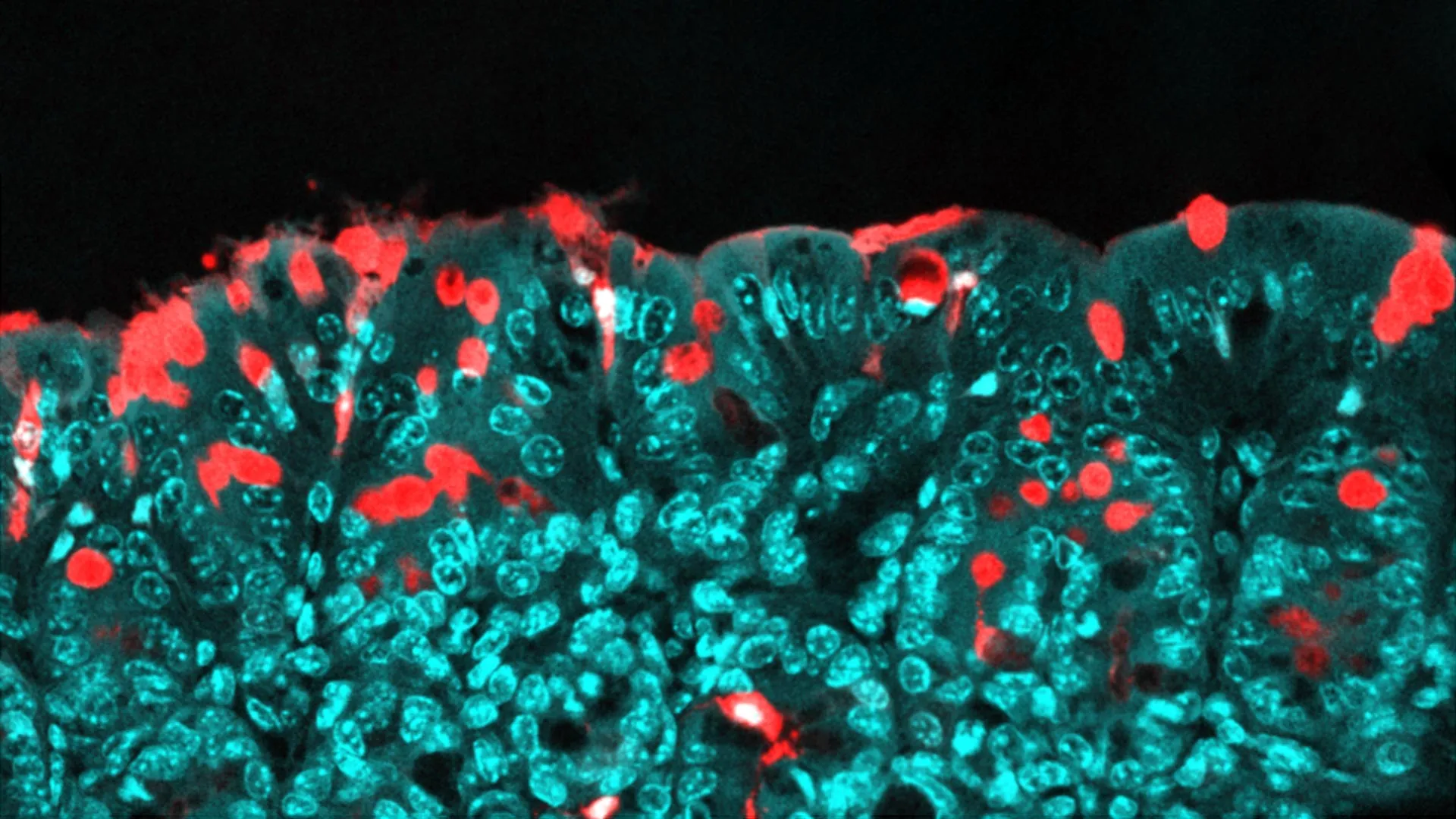
The outcomes of the investigation were striking. Within mere weeks following birth, the young mice exhibited distinct and measurable shifts in the composition and activity of their gut bacteria. This early postnatal window is recognized by developmental biologists as a profoundly sensitive and formative period for the establishment of the intestinal microbiome. During this critical time, mothers naturally transmit a significant portion of their own microbial diversity to their progeny through intimate physical contact and breast milk, a process vital for the healthy maturation of the infant’s immune system and metabolic programming. The observed alterations in the offspring’s microbial ecosystems, occurring without any direct exposure to the additives, powerfully suggested a maternal conduit for these effects.
A deeper dive into the specific changes within the offspring’s gut communities revealed an increased prevalence of certain types of bacteria characterized by flagella—whip-like appendages that enable bacterial motility. These flagellated microorganisms are well-documented for their capacity to strongly engage and stimulate the host immune system. The research team further observed a phenomenon termed "bacterial encroachment," where an abnormally high number of bacteria migrated into unusually close proximity with the epithelial lining of the gastrointestinal tract. Normally, the gut lining acts as a highly selective barrier, allowing for the controlled passage of beneficial microbial signals while excluding harmful ones.
This encroachment, spurred by the maternal emulsifier consumption, appeared to precipitate the premature closure of specific intercellular junctions, or "gut pathways," within the intestinal wall. Under normal physiological conditions, these pathways remain open for a sufficient duration during early life, facilitating a controlled interaction between nascent immune cells and minute fragments of the gut microbiota. This crucial period of "dialogue" enables the developing immune system to "learn" to distinguish between commensal (beneficial) bacteria and potential pathogens, thereby fostering a state of immune tolerance towards the body’s own resident microbial community. When these pathways sealed prematurely in the offspring of emulsifier-exposed mothers, this essential communication was profoundly disrupted, impairing the immune system’s ability to achieve proper regulatory balance.
As these animals matured into adulthood, the initial disruption in gut-immune axis communication manifested as a state of chronic immune overactivation and persistent, low-grade inflammation. This inflammatory milieu, a known precursor to various chronic ailments, significantly amplified their propensity to develop inflammatory bowel disorders—conditions like Crohn’s disease and ulcerative colitis, characterized by debilitating inflammation of the digestive tract. Furthermore, the altered metabolic signaling pathways resulting from the microbial dysbiosis and chronic inflammation also contributed to a substantially heightened risk of developing obesity, a complex metabolic disorder with far-reaching health consequences. The study thus forged a clear mechanistic link between maternal consumption of common food additives, early-life changes in offspring gut microbiota, and the subsequent development of serious chronic health conditions in adulthood, even in the absence of direct exposure to the additives by the offspring themselves.
The ramifications of these findings for human public health are considerable and warrant immediate attention. Dr. Chassaing emphasized the imperative for the scientific community to cultivate a more profound comprehension of how dietary choices made by one generation can influence the health trajectories of succeeding generations. He specifically highlighted the critical need to re-evaluate the regulatory frameworks governing food additives, particularly those incorporated into powdered infant formulas. These formulas, frequently containing emulsifiers, are often the primary source of nutrition during an exceptionally vulnerable and formative period for the infant’s gut microbiota establishment and immune system development. Direct infant exposure to such substances at this crucial juncture could compound the effects observed in the animal model.
Moving forward, the research team plans to transition their investigations into human clinical trials. These studies will focus on meticulously examining the mechanisms of mother-to-infant microbiota transmission, analyzing the impact of maternal dietary patterns—both with and without the inclusion of specific food additives—on the infant microbiome. Concurrently, they aim to study infants who are directly exposed to these emulsifying agents through the consumption of commercial baby formulas, providing invaluable insights into the direct human relevance of their animal model findings.
The complexity of the human diet, with its increasing reliance on processed foods containing a myriad of additives, presents a significant challenge for public health researchers. This study serves as a potent reminder that substances historically considered benign may exert subtle yet profound biological effects, particularly during sensitive developmental windows. Understanding these intricate interactions between diet, the microbiome, and host physiology is paramount for developing informed dietary guidelines and robust regulatory policies that safeguard public health across the lifespan and across generations. The work was made possible through financial support from a Starting Grant and a Consolidator Grant awarded by the European Research Council (ERC), underscoring the international significance and high scientific merit of this critical line of inquiry.





