For seventy years, the pharmaceutical compound hydralazine has been a cornerstone in managing critical medical conditions, primarily serving as a vital first-line intervention for severe hypertension, particularly during pregnancy. Despite its widespread and essential use, the precise molecular pathways through which hydralazine exerts its therapeutic effects have remained an enigma. This lack of detailed understanding, however, has not hindered its clinical application, but it has limited the exploration of its full therapeutic potential and the development of related, potentially superior treatments for both maternal health complications and certain types of cancer. Recent groundbreaking research has not only elucidated this long-standing mystery but has also revealed an unexpected connection between the drug’s established use and the fight against glioblastoma, an aggressive form of brain cancer.
The scientific endeavor to decipher hydralazine’s mode of action has been a significant undertaking, pursued by researchers for decades. Kyosuke Shishikura, a physician-scientist affiliated with the University of Pennsylvania, highlights the drug’s historical context, noting, "Hydralazine represents one of the earliest classes of vasodilators ever synthesized, and it continues to be a primary treatment for preeclampsia – a dangerous hypertensive disorder responsible for a substantial percentage of maternal mortality globally." He further explains that hydralazine emerged from an era of drug discovery characterized by a more empirical approach, where observed patient responses preceded a deep dive into the underlying biological mechanisms. This paradigm shift in scientific understanding has now been significantly advanced by Shishikura and his collaborators, including his postdoctoral advisor at Penn, Megan Matthews.
In a significant publication in the journal Science Advances, the research team has successfully unraveled the intricate mechanism by which hydralazine operates. Their findings have illuminated a surprising correlation between conditions involving high blood pressure, such as preeclampsia, and the cellular vulnerabilities of brain cancer. This revelation suggests that established pharmacological agents may harbor untapped therapeutic possibilities, paving the way for the design of more refined and effective medications for a spectrum of health challenges, ranging from pregnancy-related complications to oncological interventions.
Matthews emphasizes the personal and societal significance of this research, stating, "Preeclampsia has tragically affected numerous women within my own family, and its disproportionate impact on Black mothers in the United States persists." She articulates that a deeper comprehension of hydralazine’s molecular actions offers a pathway towards developing safer and more targeted therapies for hypertension during pregnancy, with the potential to markedly improve outcomes for those most susceptible to these risks.
The core of the discovery lies in identifying hydralazine’s interaction with a critical enzyme: 2-aminoethanethiol dioxygenase, or ADO. This enzyme functions as a crucial oxygen-sensing mechanism within the body, playing a pivotal role in regulating the constriction of blood vessels. The research team determined that hydralazine functions by inhibiting this oxygen-sensing enzyme.
Matthews likens the function of ADO to an immediate alert system, explaining, "ADO acts as an alarm bell that signals the moment oxygen levels begin to deplete." She elaborates on its efficiency, noting that most biological processes require time for DNA replication, RNA synthesis, and protein production. In contrast, ADO bypasses these steps, activating a biochemical switch almost instantaneously.
Hydralazine’s therapeutic action is achieved through its binding to and blockade of ADO. By effectively "muting" this oxygen-sensing alarm, hydralazine prevents the enzyme from initiating its signaling cascade. This inhibition leads to the stabilization of signaling proteins known as regulators of G-protein signaling (RGS). Shishikura explains that the accumulation of these RGS proteins signals blood vessels to cease constricting, thereby counteracting the signals that cause them to tighten. This process ultimately reduces intracellular calcium levels, which he describes as the primary determinant of vascular tone. As calcium concentrations decrease, the smooth muscle tissues in the walls of blood vessels relax, leading to vasodilation and a subsequent reduction in blood pressure.
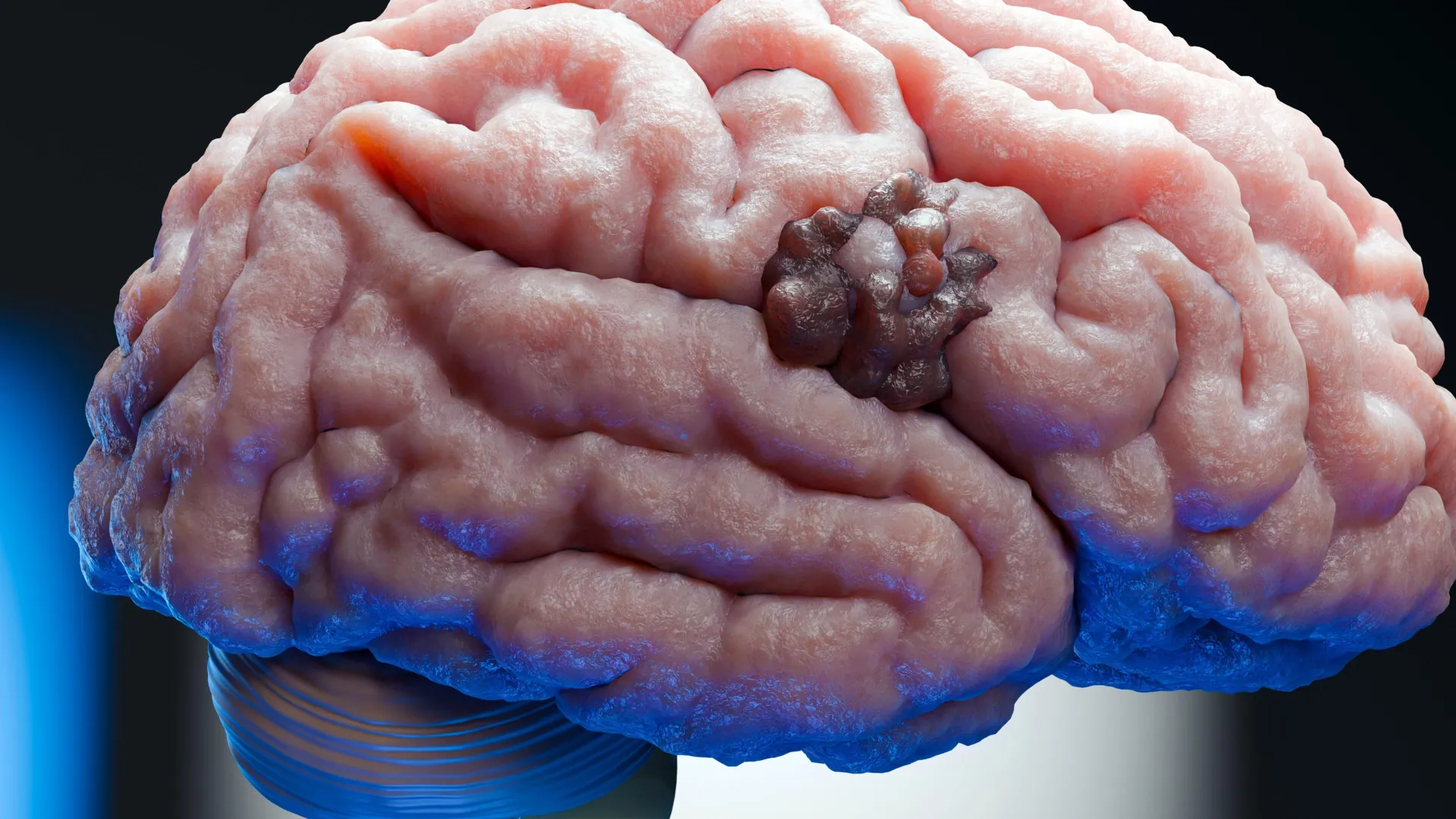
The connection between preeclampsia and brain cancer emerged as a significant, albeit unexpected, consequence of this research. Prior to this study, a growing body of evidence within the cancer research community had begun to implicate ADO in the progression of glioblastoma. Tumors of this nature frequently develop in environments characterized by severe oxygen deprivation, a condition known as hypoxia. Elevated levels of ADO and its metabolic byproducts had been correlated with more aggressive disease trajectories, suggesting that inhibiting this enzyme could be a promising therapeutic strategy. However, the absence of a potent and suitable inhibitor had hindered the direct testing of this hypothesis.
To ascertain whether hydralazine could serve as a viable candidate for this purpose, Shishikura collaborated closely with structural biochemists at the University of Texas. Utilizing X-ray crystallography, a sophisticated imaging technique, they were able to visualize hydralazine in complex with ADO’s metal center. Concurrently, neuroscientists at the University of Florida conducted experiments to evaluate the drug’s effects on brain cancer cells.
Their investigations revealed that the same ADO pathway responsible for regulating vascular contraction also plays a critical role in enabling tumor cells to survive in hypoxic conditions. Unlike conventional chemotherapy, which is designed to induce widespread cell death, hydralazine intervenes by disrupting this oxygen-sensing regulatory loop. This disruption prompts glioblastoma cells to enter a state of cellular senescence, a non-dividing, dormant phase. This effectively halts tumor growth without provoking additional inflammation or fostering resistance mechanisms, offering a novel approach to cancer therapy.
The implications of these findings extend beyond the immediate applications, underscoring the value of re-examining well-established treatments. The research demonstrates how drugs with a long history of clinical use can unlock new therapeutic avenues and contribute to the development of safer and more efficacious interventions for a range of conditions, including both maternal health disorders and oncological challenges.
The researchers are now focused on advancing this line of inquiry by synthesizing novel ADO inhibitors. The objective is to create compounds that exhibit greater tissue specificity and possess enhanced capabilities to traverse, or strategically exploit vulnerabilities within, the blood-brain barrier. Such advancements would enable targeted delivery to tumor tissues while minimizing exposure to healthy bodily systems.
Matthews is committed to continuing this pursuit of innovative medical solutions by meticulously uncovering the underlying mechanisms of established and clinically validated treatments. She remarks, "It is uncommon for a cardiovascular medication with decades of use to offer profound insights into the complexities of the brain, yet this is precisely the kind of unexpected synergy we are eager to discover – unusual connections that hold the promise of novel therapeutic breakthroughs."
The collaborative effort involved a multidisciplinary team of researchers from various institutions, including the University of Pennsylvania, the Chinese Academy of Sciences, the Georgia Institute of Technology, Nanjing Agricultural University, Oberlin College, the Pennsylvania State University, Thomas Jefferson University, the University of Florida, the University of Oxford, and Washington University in St. Louis. This extensive research was supported by significant funding from multiple national and international organizations, including the National Institutes of Health, the National Science Foundation, the American Cancer Society, the Charles E. Kaufman Foundation, the University Research Fund, the Astellas Foundation for Research on Metabolic Disorders, and the Herbert and Diane Bischoff Fund.






