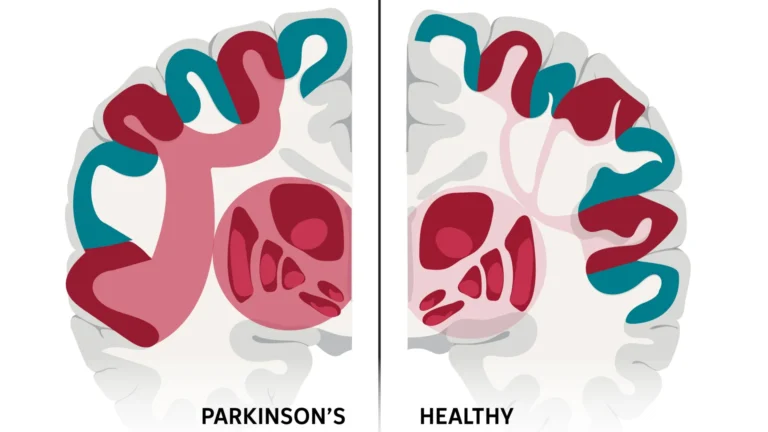
Multiple sclerosis (MS), a chronic autoimmune condition affecting approximately 2.3 million individuals globally, frequently manifests with debilitating impairments in balance and motor control. These symptoms are often linked to the progressive deterioration of the cerebellum, a critical brain region responsible for coordinating movement and maintaining equilibrium. In a significant majority of MS cases, estimated at around 80%, inflammation targets this vital area, leading to tremors, unsteadiness, and challenges in muscular regulation. The gradual loss of healthy cerebellar tissue over time exacerbates these motor deficits, profoundly impacting patients’ daily lives.
Recent groundbreaking research originating from the University of California, Riverside, has illuminated a key cellular mechanism underpinning this progressive decline in cerebellar function. Published in the esteemed journal Proceedings of the National Academy of Sciences, the study identifies a critical role for compromised mitochondria, the cellular powerhouses, in the escalating breakdown of specialized cerebellar neurons known as Purkinje cells. The observed depletion of these neurons directly correlates with the worsening motor impairments experienced by individuals with MS, offering a new lens through which to understand the disease’s progression.
The fundamental pathology of MS is characterized by persistent inflammation and the subsequent demyelination within the central nervous system. Demyelination refers to the damage or destruction of the myelin sheath, a vital protective and insulating layer that encases nerve fibers throughout the brain and spinal cord. This compromised insulation severely hampers the efficient transmission of electrical signals along nerve pathways, precipitating a wide spectrum of neurological dysfunctions, including those affecting coordination and movement.
While inflammation and myelin loss directly impair signal transmission, the UCR study highlights a parallel and equally devastating assault on the cellular energy supply. Mitochondria, responsible for generating the vast majority of a cell’s energy currency, adenosine triphosphate (ATP), are indispensable for the survival and optimal functioning of neurons. When these energy-generating organelles falter, the entire cellular machinery begins to break down.
"Our investigation, spearheaded by graduate student Kelley Atkinson under my mentorship, posits that the inflammatory and demyelinating processes occurring within the cerebellum disrupt mitochondrial integrity, thereby contributing significantly to neuronal damage and the eventual demise of Purkinje cells," stated Seema Tiwari-Woodruff, a professor of biomedical sciences at the UC Riverside School of Medicine and the lead investigator of the research team. The researchers’ examination revealed a marked reduction in COXIV, a crucial mitochondrial protein, within Purkinje cells affected by demyelination. This finding strongly suggests that mitochondrial dysfunction is not merely a consequence but a direct contributor to cell death and cerebellar pathology in MS.
The intricate dance of everyday movements, from the simple act of walking to the complex coordination required for grasping an object or maintaining an upright posture, relies on the seamless integration of signals from muscles, sensory receptors, and numerous brain regions. The cerebellum serves as the master conductor of this symphony of motor control, ensuring that movements are smooth, accurate, and well-timed.
"Within the cerebellar structure reside specialized neurons known as Purkinje cells," Professor Tiwari-Woodruff elaborated. "These are exceptionally large and metabolically active cells, playing a pivotal role in orchestrating fluid and precise movements, whether it’s executing a dance step, accurately throwing a ball, or simply navigating one’s environment. Their function is absolutely fundamental to maintaining balance and executing fine motor skills." In the context of MS and other neurodegenerative disorders affecting the cerebellum, the progressive loss of these critical neurons often results in ataxia, a debilitating condition characterized by a profound lack of coordination and marked instability in movement.
"Our examination of post-mortem cerebellar tissue from individuals diagnosed with MS revealed significant pathological changes within these crucial neurons," Professor Tiwari-Woodruff explained. "We observed a reduction in their dendritic branching, evidence of myelin loss, and critically, profound mitochondrial dysfunction, indicating a severe depletion of their energy supply. Given the central role of Purkinje cells in motor control, their degeneration inevitably leads to significant mobility challenges. Unraveling the precise reasons for their vulnerability in MS represents a vital step towards developing more effective therapeutic strategies aimed at preserving motor function and balance in affected individuals."
To gain a deeper understanding of the temporal dynamics of these cellular changes, the research team also employed experimental autoimmune encephalomyelitis (EAE), a widely utilized mouse model that recapitulates many of the key features of MS. This experimental model provided an invaluable opportunity to meticulously track the evolution of mitochondrial dysfunction as the disease progressed.
The findings from the EAE model demonstrated a consistent and progressive decline in Purkinje cell populations over time, mirroring the pathological observations in human MS patients. "The surviving neurons exhibit diminished functionality because their mitochondria, the essential energy-generating components, begin to fail," Professor Tiwari-Woodruff noted. "Furthermore, we observed that myelin breakdown occurs relatively early in the disease course. These intertwined issues—reduced cellular energy, myelin degradation, and neuronal damage—emerge early, but the actual cell death often becomes apparent later, coinciding with more severe disease manifestations. The energetic deficit within brain cells appears to be a pivotal factor driving neurodegeneration in MS."
While the EAE model does not perfectly replicate every facet of human MS, its strong parallels to the human condition make it an exceptionally powerful tool for investigating neurodegenerative processes and for evaluating the efficacy of novel therapeutic interventions.
"Our discoveries provide crucial insights into the mechanisms underlying cerebellar dysfunction in MS," Professor Tiwari-Woodruff emphasized. "The potential to target mitochondrial health offers a promising avenue for therapeutic development, with the goal of slowing or even preventing neurological deterioration and enhancing the quality of life for individuals living with MS. This research brings us measurably closer to comprehending the intricate pathological pathways of MS and to devising more potent and precisely targeted treatments for this formidable disease."
Looking ahead, the research team is actively expanding its investigations to determine whether mitochondrial dysfunction extends beyond Purkinje cells to encompass other critical cell types within the cerebellum. This includes exploring the impact on oligodendrocytes, the cells responsible for myelin production and maintenance, and astrocytes, which provide essential support to overall brain function.
"To address this question, one of our ongoing research initiatives is dedicated to scrutinizing mitochondria within specific cerebellar cell populations," Professor Tiwari-Woodruff stated. "Such research has the potential to pave the way for early neuroprotective strategies, such as enhancing cellular energy levels, facilitating myelin repair mechanisms, or modulating the immune response before irreversible damage occurs. This is particularly pertinent for individuals with MS who experience significant challenges with balance and coordination, as these symptoms are intrinsically linked to cerebellar pathology."
Professor Tiwari-Woodruff underscored the broader imperative for sustained and robust investment in scientific research. "Any reduction in funding for scientific endeavors inevitably impedes progress precisely at moments when it is most critical," she asserted. "Public support for research is, now more than ever, profoundly important." The research team responsible for this study included Shane Desfor, Micah Feria, Maria T. Sekyia, Marvellous Osunde, Sandhya Sriram, Saima Noori, Wendy Rincóna, and Britany Belloa, in addition to Atkinson and Tiwari-Woodruff. For their analysis, researchers utilized postmortem cerebellar tissue samples obtained from individuals diagnosed with secondary progressive MS, comparing them with tissue from healthy control donors. These valuable samples were sourced from the National Institutes of Health’s NeuroBioBank and the Cleveland Clinic. The study received crucial financial support from the National Multiple Sclerosis Society. The comprehensive findings are detailed in the research paper titled "Decreased mitochondrial activity in the demyelinating cerebellum of progressive multiple sclerosis and chronic EAE contributes to Purkinje cell loss."