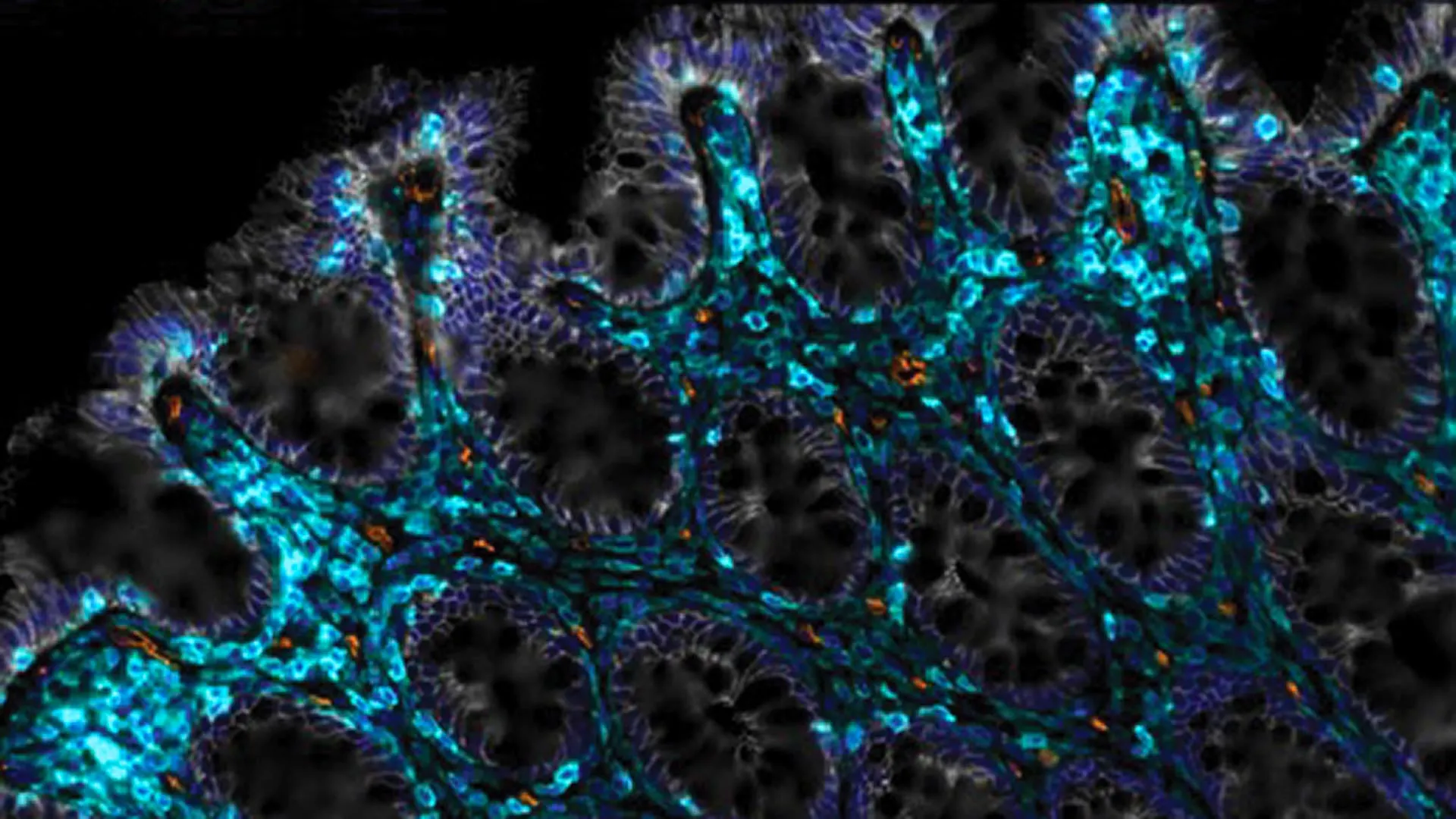
As the human body advances through its life stages, a common observation is the increasing difficulty in tolerating certain foods, a phenomenon often attributed to the gradual deterioration of the intestinal epithelium. This delicate, single-layered cellular lining plays a pivotal role in nutrient absorption and the maintenance of overall gastrointestinal well-being. Under optimal physiological conditions, this epithelial barrier undergoes a rapid and continuous cycle of renewal, typically regenerating itself every three to five days. However, the inexorable march of time, or external stressors such as radiation exposure, can significantly impede this regenerative capacity, leading to a slowdown or even a complete cessation of tissue repair. Such disruptions can foster an environment of chronic inflammation and contribute to the development of conditions like increased intestinal permeability, colloquially known as "leaky gut syndrome."
In a groundbreaking development, researchers at Cold Spring Harbor Laboratory (CSHL) have unveiled a pioneering approach designed to invigorate the intestine’s innate capacity for self-repair. Their innovative strategy leverages the power of Chimeric Antigen Receptor (CAR) T-cell therapy, a sophisticated form of immunotherapy celebrated for its efficacy in treating various forms of cancer. By adapting this potent therapeutic modality for applications within the gastrointestinal tract, the scientific team aims to pave the way for future clinical investigations focused on enhancing intestinal health, particularly for individuals experiencing age-related functional decline.
This significant advancement builds upon foundational research spearheaded by Dr. Corina Amor Vegas, an Assistant Professor at CSHL, whose laboratory has been dedicated to understanding the biological processes of cellular senescence. Cellular senescence is a state where cells, having ceased their proliferative function, persist within the body rather than undergoing programmed cell death. The accumulation of these senescent cells throughout the aging process has been implicated in the pathogenesis of a wide array of age-related ailments, including metabolic disorders like diabetes and neurodegenerative conditions such as dementia. In prior investigations, Dr. Amor Vegas and her team successfully engineered immune cells, specifically anti-uPAR CAR T cells, which exhibit a remarkable ability to selectively target and eliminate senescent cells in preclinical models. This targeted clearance resulted in substantial improvements in the metabolic profiles of the treated animals.
Building upon these promising findings, the researchers posed a critical question: could the removal of senescent cells also facilitate the restoration of the intestine’s inherent healing capabilities? To address this, Dr. Amor Vegas collaborated with Dr. Semir Beyaz, also an Assistant Professor at CSHL, and graduate student Onur Eskiocak. Their investigation involved the direct administration of engineered CAR T cells into the intestinal tracts of both young and aged mice. The outcomes, as described by Dr. Amor Vegas, were remarkably encouraging. "In both groups, we observed truly substantial improvements," she stated, highlighting enhanced nutrient absorption, a significant reduction in inflammatory markers, and a markedly accelerated rate of epithelial lining regeneration and healing in response to irritation or injury.
The therapeutic potential of this approach extends to mitigating the gastrointestinal damage often sustained by cancer patients undergoing radiation therapy, particularly in the pelvic or abdominal regions. This type of treatment frequently leads to increased intestinal permeability. To simulate this scenario, the research team subjected mice to radiation doses designed to induce damage to their intestinal epithelial cells. Crucially, mice that received the CAR T-cell treatment demonstrated a far more robust recovery compared to their untreated counterparts. A particularly noteworthy finding was the sustained positive impact of a single CAR T-cell treatment, which continued to support improved gut function for a period of at least one year.
Further underscoring the broad applicability of their findings, Mr. Eskiocak pointed to compelling evidence suggesting that anti-uPAR CAR T cells can also promote regeneration in human intestinal and colorectal cell cultures. While the precise molecular pathways underlying this regenerative effect are still under active investigation, these preliminary results strongly indicate a significant therapeutic promise. Dr. Beyaz emphasized the wider implications of this research, stating, "This represents a crucial step forward on a long and complex journey toward understanding how we can more effectively promote healing in the elderly population." This work opens new avenues for therapeutic interventions aimed at bolstering the resilience and repair mechanisms of the aging gastrointestinal system, potentially leading to improved quality of life for millions worldwide. The implications of this research extend beyond mere symptom management, hinting at the possibility of addressing the fundamental biological processes that contribute to age-related gut dysfunction. Future studies are expected to delve deeper into the specific cellular and molecular interactions that mediate this regenerative effect, potentially uncovering novel therapeutic targets for a range of gastrointestinal disorders. The successful translation of this CAR T-cell-based strategy from laboratory models to clinical application could represent a paradigm shift in the management of age-related gut decline and radiation-induced intestinal damage, offering hope for more effective and durable solutions.






