The global health landscape is profoundly shaped by the persistent challenge of obesity, with widespread recommendations advocating for weight reduction as a primary strategy to mitigate the manifold health detriments associated with excess adiposity. However, a burgeoning body of scientific inquiry is beginning to illuminate a complex interplay between age and the physiological consequences of shedding pounds, suggesting that the benefits typically associated with weight loss in younger demographics may not translate uniformly to older individuals, particularly during the critical midlife period. Emerging research indicates that for individuals in their middle years, the process of weight loss could potentially carry implications for cognitive and neural well-being, a dimension previously less emphasized in public health messaging.
A pivotal investigation undertaken by researchers at Ben-Gurion University of the Negev (BGU) sought to dissect the differential impacts of diet-induced obesity and subsequent weight reduction on the physiological systems of mice at varying life stages. The study meticulously compared the responses of young adult mice with those in their mid-aged cohort, observing the effects of a period of obesity followed by a deliberate weight loss regimen. A key finding across both age groups was the successful restoration of healthy blood glucose regulation following weight loss, underscoring the fundamental metabolic improvements that can be achieved irrespective of chronological age. This observation reinforced the established understanding that metabolic derangements associated with obesity are often reversible through weight management.
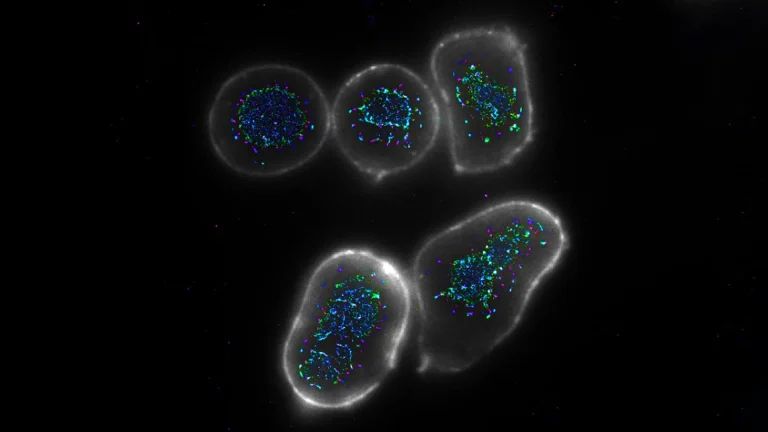
However, the researchers noted a distinct and unanticipated divergence in the mid-aged mice. While metabolic normalization occurred, their study revealed a significant escalation in inflammatory markers within the hypothalamus, a region of the brain critically involved in orchestrating a multitude of vital bodily functions, including appetite control, energy expenditure, and the maintenance of hormonal balance. This hypothalamic inflammation was not merely a superficial observation; it was meticulously documented at the molecular level, involving intricate analyses of microglia, the resident immune cells of the central nervous system. Advanced microscopic imaging techniques provided granular detail of these cellular responses, revealing an inflammatory cascade that persisted for several weeks before exhibiting a gradual abatement.
The implications of this observed brain inflammation, even if temporary, warrant careful consideration. While the precise long-term sequelae of this midlife weight-loss-induced neuroinflammation remain an open question, and it is even possible that this inflammatory response plays an intermediary role in achieving the observed metabolic benefits, the findings introduce a new layer of complexity to our understanding of aging and weight management. A substantial body of scientific literature has consistently linked chronic or inadequately managed neuroinflammation to a spectrum of cognitive impairments, including memory deficits and an increased susceptibility to neurodegenerative disorders such as Alzheimer’s disease. Therefore, this study critically prompts a re-evaluation of how interventions aimed at weight loss during midlife might intersect with, and potentially influence, the trajectory of brain health.
The lead author of the study, Alon Zemer, an M.D.-Ph.D. candidate, articulated the nuanced perspective that the research team advocates for, stating that "Our findings demonstrate that the process of losing weight during midlife is not a straightforward replication of what proves effective in younger adulthood." He further elaborated on the necessity of a more comprehensive approach, emphasizing, "While weight reduction remains an indispensable strategy for restoring metabolic equilibrium in cases of obesity, it is imperative that we deepen our comprehension of how weight loss impacts the brain in mid-aged individuals, thereby ensuring that neural well-being is not inadvertently jeopardized."
Dr. Alexandra Tsitrina, a co-author on the paper, highlighted the methodological sophistication employed in the research, noting that "Our study systematically characterizes the body’s adaptive mechanisms in response to weight loss by examining it through two complementary analytical dimensions: molecular and structural." She underscored the power of their approach, explaining that "This high-resolution imaging, facilitated by advanced microscopy and sophisticated computational analysis for image interpretation, enables the detection of subtle changes that carry significant potential health ramifications."
Moving forward, the research cohort has underscored the paramount importance of further investigations designed to elucidate the underlying mechanisms responsible for this transient yet concerning neuroinflammatory response observed during midlife weight loss. Future research endeavors are envisioned to pave the way for the development of targeted strategies that not only preserve the salutary metabolic advantages derived from weight reduction but also proactively safeguard the health of the brain as individuals navigate the aging process.
The comprehensive findings of this study, titled "Weight loss aggravates obesity-induced hypothalamic inflammation in mid-aged mice," have been formally published in the peer-reviewed journal GeroScience. The research received crucial financial backing through an internal grant awarded by BGU, specifically from the Ilse Katz Institute of Nanoscale Science and Technology. Additional support for this groundbreaking work was generously provided by grants from the U.S.-Israel Binational Science Foundation, under grant number 2021083, and the Israel Science Foundation, identified by grant number 194/24. These foundational grants have been instrumental in enabling the scientific community to explore these critical questions surrounding aging, metabolism, and brain health.